The mitogen-activated protein kinase kinase kinase kinase GCKR positively regulates canonical and noncanonical Wnt signaling in B lymphocytes - PubMed (original) (raw)
The mitogen-activated protein kinase kinase kinase kinase GCKR positively regulates canonical and noncanonical Wnt signaling in B lymphocytes
Chong-Shan Shi et al. Mol Cell Biol. 2006 Sep.
Abstract
Wnt ligands bind receptors of the Frizzled (Fz) family to control cell fate, proliferation, and polarity. Canonical Wnt/Fz signaling stabilizes beta-catenin by inactivating GSK3beta, leading to the translocation of beta-catenin to the nucleus and the activation of Wnt target genes. Noncanonical Wnt/Fz signaling activates RhoA and Rac, and the latter triggers the activation of c-Jun N-terminal kinase (JNK). Here, we show that exposure of B-lymphocytes to Wnt3a-conditioned media activates JNK and raises cytosolic beta-catenin levels. Both the Rac guanine nucleotide exchange factor Asef and the mitogen-activated protein kinase kinase kinase kinase germinal center kinase-related enzyme (GCKR) are required for Wnt-mediated JNK activation in B cells. In addition, we show that GCKR positively affects the beta-catenin pathway in B cells. Reduction of GCKR expression inhibits Wnt3a-induced phosphorylation of GSK3beta at serine 9 and decreases the accumulation of cytosolic beta-catenin. Furthermore, Wnt signaling induces an interaction between GCKR and GSK3beta. Our findings demonstrate that GCKR facilitates both canonical and noncanonical Wnt signaling in B lymphocytes.
Figures
FIG. 1.
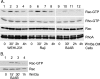
Wnt3a-conditioned media (Wnt3a CM) or a recombinant mouse Wnt3a ligand induces Rac but not RhoA activation in B-cell lines. (A) Wnt3a CM induces Rac but not RhoA activation in three B cell lines. WEHI-231 cells, Raji, and BJAB B-cell lines were exposed to Wnt3a CM, and Rac and RhoA activation was measured in cell lysates prepared at various time points following exposure. The levels of GTP-bound Rac and RhoA were determined by pulldown assays and subsequent immunoblotting for Rac (top row) and RhoA (second row). The third and fourth rows show the levels of Rac and RhoA proteins in cell lysates prepared from the WEHI-231, Raji, and BJAB B-cell lines. The anti-Rac antibodies recognize Rac1 and Rac2, while the RhoA antibody recognizes RhoA, RhoB, and RhoC. Experiments were performed three times, with similar results. (B) A recombinant mouse Wnt3a ligand induces Rac activation in the BJAB B-cell line. The BJAB cells were treated with Wnt3a ligand (20 ng/ml) for the indicated times. The GTP-bound Rac was detected by a pulldown assay. The first panel shows the GTP-Rac levels, and the second shows total Rac protein levels.
FIG. 2.
Wnt3a-conditioned medium (Wnt3a CM) stimulates GCKR kinase activity and JNK phosphorylation and protects β-catenin from degradation. (A) WEHI-231, Raji, and BJAB B-cell lines were stimulated with Wnt3a CM for 30 min, 2 h, and 4 h. An in vitro kinase assay was used to determine the level of GCKR kinase activity, and JNK activation was assessed by immunoblotting with phospho-specific JNK antibodies. The amounts of JNK1/JNK2 expression in the cell lysates were assessed by immunoblotting with JNK-specific antibodies and are shown. Two experiments were performed, with similar results. (B) sFRP-1 specifically impairs Wnt3a ligand-induced GCKR kinase activity and JNK phosphorylation. Wnt3a CM was incubated with sFRP-1 (100 ng/ml) for 1/2 hour to neutralize Wnt3a ligand in the conditioned media; subsequently, the media with sFRP-1 were used to treat Raji and BJAB cells. Wnt3a CM without sFRP-1 was used to treat the cells for 0 minutes as a basal control and for 30 min as a positive control. Myelin basic protein was used as a substrate in a GCKR in vitro kinase assay. An immunoblot of GCKR from immunoprecipitated GCKR used in the in vitro kinase assay is shown. Cell lysates were immunoblotted for phospho-JNK (pJNK) and total JNK. (C) Wnt3a CM also stimulate the accumulation of β-catenin. The levels of β-catenin and β-actin, which served as a loading control, were determined by immunoblotting of the cell lysates prepared as described above. Three experiments were performed, with similar results.
FIG. 3.
Silencing GCKR expression in BJAB and Raji cells impairs JNK activation. BJAB (A) and Raji (B) cells were stably transfected with an shRNA construct designed to interfere with GCKR expression or a control construct. The indicated cell lines were treated with Wnt3a-conditioned media (Wnt3a CM) for 30 min, 2 h, and 4 h. Cell lysates were immunoblotted for GCKR, β-actin, phospho-JNK (pJNK), and JNK1/JNK2. Two experiments were performed, with similar results.
FIG. 4.
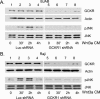
Wnt3a activates Rac via Asef and APC, and Rac activation is required for Wnt3a-induced GCKR and JNK activation. (A) Effect of silencing APC or Asef expression on Wnt3a-induced Rac and JNK activation. Raji B cells were stably transfected with shRNA constructs targeted at APC or Asef or with a control shRNA construct. The various cell lines were stimulated with Wnt3a-conditioned media (Wnt3a CM) and the cell lysates subjected to a GTP-Rac pulldown assay and immunoblotted for the amount of Rac, phospho-JNK (pJNK), or JNK1/JNK2. Two experiments were performed, with similar results. (B) Wnt3a-induced GCKR activation depends upon Rac activation. Raji cells were stably transfected with constructs expressing either Rac-N17, Asef shRNA, APC shRNA, or a control shRNA and were stimulated with Wnt3a CM for various durations. GCKR immunoprecipitates were subjected to an in vitro kinase assay, using myelin basic protein as a substrate. Each blot was exposed for a similar duration. To verify that equivalent amounts of GCKR were immunoprecipitated, the upper portion of the gel for the kinase assay was removed and immunoblotted for GCKR expression. Each experiment was performed at least twice. (C) Verification that APC and Asef silencing reduce protein expression. Cell lysates from the Raji cells transfected with constructs expressing either APC or Asef shRNAs were subjected to immunoblotting with either APC- or Asef-specific antiserum.
FIG. 5.
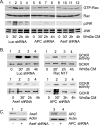
Role for GCKR in noncanonical and canonical Wnt signaling. (A) _Gckr_−/−progenitor B cells are defective in Wnt3a-induced signaling pathways. Bone marrow-derived progenitor B cells from wild-type and _Gckr_−/− mice were treated with Wnt3a-conditioned media (Wnt3a CM) for various durations and cell lysates prepared. Immunoblots show GCKR, phospho-JNK (pJNK), JNK1/JNK2, and β-catenin levels in the cell lysates. Each blot was exposed for a similar duration. Two experiments were performed, with similar results. (B) Expression of GCKR in 293 cells activates a TCF reporter. Super8XTOPFlash (15 ng) or Super8XFOPFlash (control) were transfected along with a construct expressing either GCKR (100 ng) or a kinase-dead form of GCKR (GCKR-KD; 100 ng). Twenty-four hours later, cell lysates were prepared and luciferase activities measured. The averages and standard deviations for the inductions obtained from triplicate determinations are shown. Experiments were performed a minimum of four times, with similar results. Immunoblots show GCKR, phospho-JNK, and JNK in the cell lysates from the SuperXTOPFlash-transfected cells (lanes 1 to 3). (C) Reducing GCKR expression in 293 cells impairs Wnt3a-induced TCF reporter gene activation. Super8XTOPFlash or Super8XFOPFlash luciferase reporter genes were transfected along with constructs expressing an shRNA targeted at GCKR or GFP. Following the transfection, Wnt3a (30 ng) was added or not added. Twenty-four hours later, cell lysates were prepared and luciferase activities measured. The averages and standard deviations for the inductions of luciferase activities obtained from triplicate determinations are shown. The experiment was performed twice, with similar results. Immunoblots show GCKR, phospho-JNK, and JNK in the cell lysates from the Super8XTOPflash transfections (lanes 1 to 4). (D) GCKR is important for the stabilization of cytoplasmic β-catenin. Constructs expressing GCKR, kinase-dead GCKR, GCKR shRNA, and GFP shRNA were transfected into 293 cells, and Wnt3a (30 ng) was added or not added. The cytoplasmic fraction and cellular membranes were separated. Immunoblots show β-catenin levels (cytoplasmic, membrane associated, and presumably ubiquitinated) and GCKR levels (total cell lysate). The Ub-β-catenin immunoblot shown is a long exposure of the upper portion (above 100 kDa) of the cytoplasmic β-catenin immunoblot. The experiment was performed twice, with similar results.
FIG. 6.
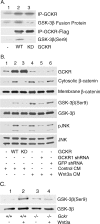
Involvement of GCKR in the phosphorylation of GSK3β. (A) GCKR phosphorylates an N-terminal GSK3 peptide, and a portion is on serine 9 (Ser9). Constructs expressing GCKR or a kinase-dead form were transfected into 293 cells, and GCKR immunoprecipitates were used in an in vitro kinase assay using as a substrate a 20-amino-acid peptide that spans serines 9 and 21 in the N-terminal portion of GSK3β fused to GST. Shown are the levels of GCKR in the immunoprecipitates (top row) and the phosphorylated fusion protein (second row). Endogenous GCKR in 293 cells accounts for the band in lane 1. Also, Flag empty vector, Flag-GCKR, and Flag-GCKR KD (kinase dead) were transfected into 293 cells. Bead-linked anti-flag antibodies were used to immunoprecipitate the expressed proteins. After being washed six times, the beads were incubated with GST-GSK3 peptide in reaction buffer for 30 min. The third row shows the immunoprecipitated (IP) Flag GCKR. The fourth row shows the serine 9 phosphorylation of GSK3 with a phospho-specific antibody. (B) GCKR is important for serine 9 phosphorylation of GSK3β in 293 cells. Constructs expressing GCKR, the GCKR kinase-dead form, GCKR shRNA, and GFP shRNA were transfected into 293 cells. Media obtained from 293 cells transfected with a control construct or a Wnt3a expression construct were used to treat the cells shown in lanes 1 to 3 and 4 to 6, respectively, for 30 min prior to cell lysis. Immunoblots of cell lysates for GCKR, phosphorylated (serine 9) and total GSK3β, and phosphorylated (pJNK) and total JNK are shown. In addition, a portion of each cell lysate was fractionated into cytosolic and membrane fractions and immunoblotted for β-catenin. WT, wild type. (C) GCKR is important for serine 9 phosphorylation of GSK3β in progenitor B cells. Bone marrow-derived progenitor B cells from wild-type and _Gckr_−/− mice were prepared and stimulated with Wnt3a for 15 min. Immunoblots of cell lysates for phosphorylated (serine 9) and total GSK3β are shown. All experiments were performed at least twice, with similar results.
FIG. 7.
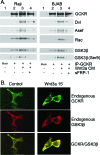
Wnt3a enhances the association of GCKR with Dvl, Asef, Rac, and GSK3β. (A) Wnt3a-conditioned media augment the immunoprecipitation of GCKR with Dvl, Asef, Rac, and GSK3β. Raji and BJAB B-cell lines were treated with Wnt3a-conditioned media for 30 min, or sFRP-1 (100 ng/ml) was incubated with Wnt3a-conditioned media for 1/2 hour to neutralize the activity of the Wnt3a ligand before treatment of the cells. The GCKR immunoprecipitates prepared from the cell lysates were immunoblotted for the presence of Dvl, Asef, and Rac proteins. The same GCKR immunoprecipitates were also examined for the presence of GSK3β and serine 9 (Ser9)-phosphorylated GSK3β. Two experiments were performed, with similar results. (B) Wnt3a enhances colocalization of endogenous GCKR with GSK3β. 293 cells were treated with Wnt3a ligand (15 ng/ml) for 15 min. The cells were fixed and stained with antibodies reactive with GCKR (green) and GSK3β (red). Colocalization of GCKR and GSK3β is indicated by yellow.
FIG. 8.
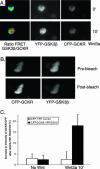
Further evidence that Wnt signaling triggers an interaction between GCKR and GSK3β. (A) FRET reveals a Wnt-induced interaction between GKCR and GSK3β in live cells. Constructs expressing CFP-GCKR, YFP-GSK3β, or both were transfected into 293 cells. The following day, the cells were incubated in media without serum for 4 h and transferred to the microscope stage, where they were maintained at 37°C with 5% CO2, and Wnt3a (20 ng/ml) was added, during which time the cells were imaged. We briefly illuminated the cells with a 436-nm laser line and simultaneously measured the emission of CFP and YPF and the spillover-corrected _F_YFP/_F_CFP ratio. Shown are images simultaneously acquired in the CFP and YFP channels at time zero and 10 min after addition of Wnt3a ligand and the corrected ratio images. The YFP images and the YFP-GSK/CFP-GCKR emission ratio images in response to Wnt3a (20 ng/ml) are shown. The color scale goes from green (no FRET) to yellow to red (high FRET). Similar results were obtained in three experiments. (B) Donor dequenching after acceptor photobleaching confirms an interaction between GCKR and GSK3β. 293 cells were transfected with CFP-GCKR and YFP-GSK3β for 24 h, after which the cells were incubated for 4 h without serum media. Before being fixed, the cells were treated with Wnt3a (20 ng/ml) for 10 min. The cells were illuminated with a 436-nm laser line, and images in the CFP and YFP emission ranges were acquired. Thereafter, the YFP was photobleached and a second CFP image was acquired. Note the increase in the intensity of the CFP image following photobleaching. Experiments were performed four times, with similar results. (C) Quantification of the increase in the intensity of CFP-GCKR after acceptor photobleaching. 293 cells were transfected with CFP-GCKR and YFP-GSK3β for 24 h, after which the cells were incubated for 4 h without serum media. Before being fixed, the cells were treated with Wnt3a (20 ng/ml) for 10 min or left untreated. A minimum of five cells was analyzed in each of three different experiments. As a control, similar experiments were performed on cells transfected with constructs that express CFP and YFP.
Similar articles
- p38 mitogen-activated protein kinase regulates canonical Wnt-beta-catenin signaling by inactivation of GSK3beta.
Bikkavilli RK, Feigin ME, Malbon CC. Bikkavilli RK, et al. J Cell Sci. 2008 Nov 1;121(Pt 21):3598-607. doi: 10.1242/jcs.032854. J Cell Sci. 2008. PMID: 18946023 - Modulation of Wnt3a-mediated nuclear beta-catenin accumulation and activation by integrin-linked kinase in mammalian cells.
Oloumi A, Syam S, Dedhar S. Oloumi A, et al. Oncogene. 2006 Dec 14;25(59):7747-57. doi: 10.1038/sj.onc.1209752. Epub 2006 Jun 26. Oncogene. 2006. PMID: 16799642 - Frat oncoproteins act at the crossroad of canonical and noncanonical Wnt-signaling pathways.
van Amerongen R, Nawijn MC, Lambooij JP, Proost N, Jonkers J, Berns A. van Amerongen R, et al. Oncogene. 2010 Jan 7;29(1):93-104. doi: 10.1038/onc.2009.310. Epub 2009 Oct 5. Oncogene. 2010. PMID: 19802005 - Dishevelled: The hub of Wnt signaling.
Gao C, Chen YG. Gao C, et al. Cell Signal. 2010 May;22(5):717-27. doi: 10.1016/j.cellsig.2009.11.021. Epub 2009 Dec 13. Cell Signal. 2010. PMID: 20006983 Review. - [Dual-role regulations of canonical Wnt/beta-catenin signaling pathway].
Liu Y, Zhang CG, Zhou CY. Liu Y, et al. Beijing Da Xue Xue Bao Yi Xue Ban. 2010 Apr 18;42(2):238-42. Beijing Da Xue Xue Bao Yi Xue Ban. 2010. PMID: 20396373 Review. Chinese.
Cited by
- β-catenin promotes the type I IFN synthesis and the IFN-dependent signaling response but is suppressed by influenza A virus-induced RIG-I/NF-κB signaling.
Hillesheim A, Nordhoff C, Boergeling Y, Ludwig S, Wixler V. Hillesheim A, et al. Cell Commun Signal. 2014 Apr 26;12:29. doi: 10.1186/1478-811X-12-29. Cell Commun Signal. 2014. PMID: 24767605 Free PMC article. - A Wnt survival guide: from flies to human disease.
Chien AJ, Conrad WH, Moon RT. Chien AJ, et al. J Invest Dermatol. 2009 Jul;129(7):1614-27. doi: 10.1038/jid.2008.445. Epub 2009 Jan 29. J Invest Dermatol. 2009. PMID: 19177135 Free PMC article. Review. - PPE38 of Mycobacterium marinum triggers the cross-talk of multiple pathways involved in the host response, as revealed by subcellular quantitative proteomics.
Wang H, Dong D, Tang S, Chen X, Gao Q. Wang H, et al. J Proteome Res. 2013 May 3;12(5):2055-66. doi: 10.1021/pr301017e. Epub 2013 Apr 8. J Proteome Res. 2013. PMID: 23514422 Free PMC article. - Mitogen-activated protein kinases and Wnt/beta-catenin signaling: Molecular conversations among signaling pathways.
Bikkavilli RK, Malbon CC. Bikkavilli RK, et al. Commun Integr Biol. 2009;2(1):46-9. doi: 10.4161/cib.2.1.7503. Commun Integr Biol. 2009. PMID: 19513264 Free PMC article. - β-Catenin is central to DUX4-driven network rewiring in facioscapulohumeral muscular dystrophy.
Banerji CR, Knopp P, Moyle LA, Severini S, Orrell RW, Teschendorff AE, Zammit PS. Banerji CR, et al. J R Soc Interface. 2015 Jan 6;12(102):20140797. doi: 10.1098/rsif.2014.0797. J R Soc Interface. 2015. PMID: 25551153 Free PMC article.
References
- Angers, S., C. J. Thorpe, T. L. Biechele, S. J. Goldenberg, N. Zheng, M. J. Maccoss, and R. T. Moon. 2006. The KLHL12-Cullin-3 ubiquitin ligase negatively regulates the Wnt-beta-catenin pathway by targeting Dishevelled for degradation. Nat. Cell Biol. 8:348-357. - PubMed
- Behrens, J., J. P. von Kries, M. Kuhl, L. Bruhn, D. Wedlich, R. Grosschedl, and W. Birchmeier. 1996. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature 382:638-642. - PubMed
- Boutros, M., N. Paricio, D. I. Strutt, and M. Mlodzik. 1998. Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell 94:109-118. - PubMed
- Chin, A. I., J. Shu, C. Shan Shi, Z. Yao, J. H. Kehrl, and G. Cheng. 1999. TANK potentiates tumor necrosis factor receptor-associated factor-mediated c-Jun N-terminal kinase/stress-activated protein kinase activation through the germinal center kinase pathway. Mol. Cell. Biol. 19:6665-6672. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous