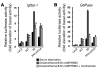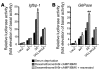The LXXLL motif of murine forkhead transcription factor FoxO1 mediates Sirt1-dependent transcriptional activity - PubMed (original) (raw)
. 2006 Sep;116(9):2473-83.
doi: 10.1172/JCI25518. Epub 2006 Aug 17.
Affiliations
- PMID: 16917544
- PMCID: PMC1550275
- DOI: 10.1172/JCI25518
The LXXLL motif of murine forkhead transcription factor FoxO1 mediates Sirt1-dependent transcriptional activity
Jun Nakae et al. J Clin Invest. 2006 Sep.
Abstract
The forkhead transcription factor FoxO1 has been identified as a negative regulator of insulin/IGF-1 signaling. Its function is inhibited by phosphorylation and nuclear exclusion through a PI3K-dependent pathway. However, the structure/function relationship of FoxO1 has not been elucidated completely. In this study, we carried out mutation analysis of the FoxO1 coactivator-interacting LXXLL motif (amino acids 459-463). Expression of a 3A/LXXAA mutant, in which 3 Akt phosphorylation sites (T24, S253, and S316) and 2 leucine residues in the LXXLL motif (L462 and L463) were replaced by alanine, decreased both Igfbp-1 and G6Pase promoter activity and endogenous Igfbp-1 and G6Pase gene expression in simian virus 40-transformed (SV40-transformed) hepatocytes. Importantly, mutagenesis of the LXXLL motif eliminated FoxO1 interaction with the nicotinamide adenine dinucleotide-dependent (NAD-dependent) deacetylase sirtuin 1 (Sirt1), sustained the acetylated state of FoxO1, and made FoxO1 nicotinamide and resveratrol insensitive, supporting a role for this motif in Sirt1 binding. Furthermore, intravenous administration of adenovirus encoding 3A/LXXAA FoxO1 into Lepr db/db mice decreased fasting blood glucose levels and improved glucose tolerance and was accompanied by reduced G6Pase and Igfbp-1 gene expression and increased hepatic glycogen content. In conclusion, the LXXLL motif of FoxO1 may have an important role for its transcriptional activity and Sirt1 binding and should be a target site for regulation of gene expression of FoxO1 target genes and glucose metabolism in vivo.
Figures
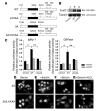
Figure 1. Scheme and subcellular localization of FoxO1 mutants in SV40-transformed hepatocytes and expression of an Igfbp-1 /luciferase or G6Pase/luciferase reporter gene in SV40-transformed hepatocytes transfected with WT or LXXAA FoxO1.
(A) Scheme of WT, LXXAA FoxO1, 3A FoxO1, and 3A/LXXAA FoxO1. (B) Expression of cMyc-WT (lane 2) and LXXAA FoxO1 (lane 3) in SV40-transformed hepatocytes was detected. (C) After overnight serum deprivation and induction with dexamethasone, 8-Br-cAMP, and IBMX in the presence or absence of insulin (100 nM) as described in Methods, cells were harvested and luciferase activity was measured. *Statistically significant difference between control and WT-transfected cells, P < 0.001 by 1-way ANOVA; **statistically significant difference between WT-and LXXAA-transfected cells, P < 0.005 by 1-way ANOVA. Data represent mean ± SEM from 3 independent transfection experiments. Following transient transfection, hepatocytes were seeded onto 4-well slide culture chambers and incubated in various conditions, including serum deprivation, for 24 hours (D), stimulation with insulin (100 nM, 20 minutes) (E), incubation with serum (F), and stimulation with H2O2 (250 μM, 4 hours) (G). Myc epitope–tagged FoxO1 was visualized with anti-cMyc monoclonal antibody and FITC-conjugated anti-mouse IgG.
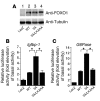
Figure 2. Expression of an Igfbp-1 /luciferase or G6Pase /luciferase reporter gene in SV40-transformed hepatocytes transduced with adenovirus encoding LacZ, WT FoxO1, 3A FoxO1, or 3A/LXXAA FoxO1.
(A) Expression of HA-WT FoxO1, Flag 3A FoxO1, or 3A/LXXAA FoxO1 in SV40-transformed hepatocytes was detected. Lane 1, hepatocytes transduced with adenovirus encoding LacZ; lane 2, with WT; lane 3, with 3A FoxO1; lane 4, with 3A/LXXAA FoxO1 at 10 MOI. After hepatocytes were transfected with Igfbp-1/(p925GL3) (B) or G6Pase/(PicaGene/ human G6Pase promoter-luciferase) (C), cells were infected with indicated adenovirus. Synthetic Renilla luciferase reporter vector (phRL-SV40) was used as an internal control of transfection efficiency. After overnight serum deprivation and induction with dexamethasone, 8-Br-cAMP, and IBMX as described in Methods, cells were harvested and luciferase activity was measured. *Statistically significant difference between 3A FoxO1– and WT FoxO1– or 3A/LXXAA FoxO1–transduced cells, P < 0.001 by 1-way ANOVA. Data represent mean ± SEM from 3 independent experiments.
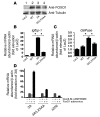
Figure 3. Effects of 3A FoxO1 and 3A/LXXAA FoxO1 mutants on endogenous Igfbp-1 or G6Pase gene expression in SV40-transformed hepatocytes.
(A) Expression of HA-WT, Flag 3A FoxO1, or 3A/LXXAA FoxO1 in SV40-transformed hepatocytes was detected. Lane 1, hepatocytes transduced with adenovirus encoding LacZ; lane 2, with WT; lane 3, with 3A mutant; lane 4, with 3A/LXXAA mutant at 10 MOI. Endogenous Igfbp-1 (B) or G6Pase (C) gene expression in SV40-transformed hepatocytes transduced with adenovirus encoding LacZ, WT FoxO1, 3A FoxO1, or 3A/LXXAA FoxO1. Hepatocytes transduced with each adenovirus were incubated in serum-free medium overnight before addition of dexamethasone/8-Br-cAMP/IBMX and incubation for 8 hours. Isolation of total RNA and real-time PCR were performed as described in Methods. Data (mean ± SEM) are from 3 independent experiments and are normalized by the amount of β_-actin_ mRNA and expressed relative to the corresponding LacZ value. *Statistically significant difference between 3A- and WT- or 3A/LXXAA-transduced cells at each MOI (P < 0.001 by 1-way ANOVA). (D) Effects of 3A FoxO1, 3A/LXXAA FoxO1, and Δ256 FoxO1 mutants on endogenous Igfbp-1 gene expression in SV40-transformed hepatocytes. Hepatocytes transduced with each adenovirus at 10 MOI were incubated in serum-free medium overnight before addition of dexamethasone/8-Br-cAMP/IBMX for 8 hours. Isolation of total RNA and real-time PCR were performed as described in Methods. Data are from 3 independent experiments and are expressed as mean ± SEM of fold of relative gene expression for β-actin in LacZ-transduced hepatocytes in the absence of dexamethasone/8-Br-cAMP/IBMX.
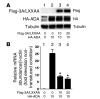
Figure 4. Effects of 3A/LXXAA FoxO1 on endogenous Igfbp-1 gene expression in SV40-transformed hepatocytes.
(A) Expression of Flag 3A/LXXAA FoxO1 and HA-ADA FoxO1 at indicated MOI in SV40-transformed hepatocytes. (B) The effects of 3A/LXXAA FoxO1 on HA-ADA–induced Igfbp-1 gene expression in the presence of dexamethasone/8-Br-cAMP/IBMX in SV40-transformed hepatocytes. Data from 3 independent experiments are expressed as mean ± SEM of the folds of endogenous Igfbp-1 gene expression in the absence of HA-ADA FoxO1. *Statistically significant difference between 3A/LXXAA FoxO1 at MOI 0 and at MOI 10 or 20. P < 0.001 by 1-way ANOVA.
Figure 5. Sirt1 interacts with 3A FoxO1 but not with 3A/LXXAA FoxO1, and disruption of the LXXLL motif enhances acetylation of FoxO1.
(A) SV40-transformed hepatocytes were transduced with adenovirus encoding Flag 3A FoxO1 (lane 1) or Flag 3A/LXXAA FoxO1 (lane 2). After 24 hours serum starvation, cells were cultured in AMEM supplemented with 0.1% bovine serum albumin, 0.5 mM 8-Br-cAMP, 0.5 mM IBMX, and 1 μM dexamethasone for 8 hours. Lysates were immunoprecipitated using anti-Flag monoclonal antibody (M2) and Western blotted with anti-Sir2 polyclonal (first panel) or anti-Flag monoclonal antibody (M2) (fourth panel) or immunoprecipitated using anti-Sir2 polyclonal antibody and Western blotted with anti-Sir2 antibody (second panel) or anti-Flag monoclonal antibody (third panel). (B) SV40-transformed hepatocytes transduced with adenovirus encoding Flag 3A FoxO1 (lane 1) or Flag 3A/LXXAA FoxO1 (lane 2) were incubated with 0.5 mM 8-Br-cAMP, 0.5 mM IBMX, and 1 μM dexamethasone for 8 hours after 24 hours of serum starvation. Lysates were immunoprecipitated using anti-Flag monoclonal antibody (M2) and Western blotted with anti-acetylated lysine polyclonal (top panel) or anti-Flag monoclonal antibody (M2) (bottom panel). (C) Quantification of the data in B. Mean ± SEM of the folds of acetylation of 3A/LXXAA was calculated from 3 independent experiments using NIH Image 1.62 (http://rsb.info.nih.gov/nih-image/). Subsequent blotting with anti-Flag antibody using the same filter normalized acetylation of each FoxO1. (D) HEK293 cells transfected with pCMV5/cMyc/3A FoxO1 (lanes 1–4) or 3A/LXXAA FoxO1 (lanes 5–8) were incubated for 8 hours in the absence or presence of nicotinamide (50 mM) or trichostatin A (TSA) (2 μM). cMyc-tagged FoxO1 was immunoprecipitated, and the acetylation of FoxO1 was assessed by Western blot with the antibody to acetylated lysine (upper panel). Total levels of FoxO1 were assessed with the antibody to cMyc (lower panel).
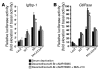
Figure 6. Nicotinamide inhibits FoxO1-dependent induction of a reporter construct under the control of an Igfbp-1 or G6Pase promoter.
After hepatocytes were transfected with Igfbp-1/(p925GL3) (A) or G6Pase/luciferase reporter gene (PicaGene/human G6Pase promoter-luciferase) (B), cells were infected with indicated adenovirus. phRL-SV40 was used as an internal control of transfection efficiency. After overnight serum deprivation (black bars) and induction with dexamethasone/8-Br-cAMP/ IBMX as described in Methods in the presence (light gray bars) or absence (dark gray bars) of nicotinamide (50 mM), cells were harvested and luciferase activity was measured. *Statistically significant difference between luciferase activity in the absence and in the presence of nicotinamide in cells transfected with each vector. P < 0.005 by 1-way ANOVA. Data represent mean ± SEM from 3 independent experiments.
Figure 7. BML-210 inhibits FoxO1-dependent induction of a reporter construct under the control of an Igfbp-1 or G6Pase promoter.
After hepatocytes were transfected with Igfbp-1/(p925GL3) (A) or G6Pase/luciferase reporter gene (PicaGene/human G6Pase promoter-luciferase) (B), cells were infected with indicated adenovirus. phRL-SV40 was used as an internal control of transfection efficiency. After overnight serum deprivation (black bars) and induction with dexamethasone/8-Br-cAMP/IBMX as described in Methods in the presence (light gray bars) or absence (dark gray bars) of BML-210 (1.5 mM), cells were harvested and luciferase activity was measured. *Statistically significant difference between luciferase activity in the absence and luciferase activity in the presence of BML-210 at cells transfected with each vector, P < 0.01 by 1-way ANOVA. Data represent mean ± SEM from 3 independent experiments.
Figure 8. Resveratrol activates induction of a reporter construct under the control of an Igfbp-1 or G6Pase promoter.
After hepatocytes were transfected with Igfbp-1/(p925GL3) (A) or G6Pase/luciferase reporter gene (PicaGene/human G6Pase promoter-luciferase) (B), cells were infected with indicated adenovirus. phRL-SV40 was used as an internal control of transfection efficiency. After overnight serum deprivation (black bars) and induction with dexamethasone/8-Br-cAMP/IBMX as described in Methods in the presence (light gray bars) or absence (dark gray bars) of resveratrol (10 μM), cells were harvested and luciferase activity was measured. *Statistically significant difference between luciferase activity in the absence and luciferase activity in the presence of resveratrol, *P < 0.005 and **P < 0.001 by 1-way ANOVA. Data represent mean ± SEM from 3 independent experiments.
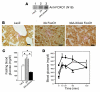
Figure 9. Effects of overexpression of 3A/LXXAA FoxO1 on fasted blood glucose and glucose tolerance in _Lepr_db/db mice.
(A) Expression of 3A FoxO1 or 3A/LXXAA FoxO1 mutant in liver. Liver was removed from mice at day 5 after injection with adenovirus encoding LacZ (lane 1), 3A FoxO1 (lane 2), or 3A/LXXAA FoxO1 (lane 3) intravenously, and 15 μg of each liver lysate was subjected to immunoblot analysis with an anti-FOXO1 polyclonal antibody (N18). (B) Immunohistochemistry of liver from _Lepr_db/db mice injected with adenovirus encoding LacZ (left panel), 3A FoxO1 (middle panel), or 3A/LXXAA FoxO1 (right panel). Each panel shows representative sections. Magnification, ×40. Scale bar: 10 μm. (C) Effect of overexpression of 3A FoxO1 or 3A/LXXAA FoxO1 in liver from _Lepr_db/db mice on fasted blood glucose. Blood glucose levels were measured as described in Methods after overnight fasting at day 5 after injection with each adenovirus encoding LacZ (white bar), 3A FoxO1 (gray bar) or 3A/LXXAA FoxO1 (black bar). Data represent mean ± SEM of 6 mice for each group. *Statistically significant difference between mice injected with each adenovirus encoding LacZ, 3A FoxO1, or 3A/LXXAA FoxO1. P < 0.001 by 1-way ANOVA. (D) Intraperitoneal glucose tolerance test was performed in mice injected with the adenoviruses indicated. The groups of mice indicated were injected with a 1.2 g/kg of body weight of glucose intraperitoneally as follows: LacZ (open circles, n = 6), 3A FoxO1 (filled circles, n = 6), and 3A/LXXAA FoxO1 (filled squares, n = 6). *Statistically significant difference between 3A and 3A/LXXAA, P < 0.01 by 1-way ANOVA; **statistically significant difference between 3A/LXXAA and LacZ or 3A, P < 0.01 by 1-way ANOVA.
Figure 10. Effects of overexpression of 3A/LXXAA FoxO1 in liver on gene expression and hepatic glycogen content.
(A) Real-time PCR. Following overnight fasting, mRNA from the liver of _Lepr_db/db mice injected with adenovirus encoding LacZ (white bars), 3A FoxO1 (gray bars), or 3A/LXXAA FoxO1 (black bars) was isolated at day 5 after injection. Real-time PCR was performed with primers encoding the genes indicated. Data represent mean ± SEM of 3 independent experiments (n = 4 for each group). *Statistically significant difference between 3A FoxO1 and 3A/LXXAA FoxO1, P < 0.02 by 1-way ANOVA; **statistically significant difference between LacZ and 3A FoxO1, P < 0.05 by 1-way ANOVA. (B) ChIP assay of Igfbp-1 (lanes 1–3), G6Pase (lanes 4–6), and Pepck (lanes 7–9) promoter. ChIP assay was performed using liver sections from _Lepr_db/db mice injected with adenovirus encoding LacZ, 3A FoxO1, or 3A/LXXAA FoxO1 with the indicated antibody. The cross-linked DNA was amplified by PCR using a set of primers spanning the forkhead binding sites in the Igfbp-1, G6Pase, or Pepck promoter. (C) Hepatic glycogen content. _Lepr_db/db mice at day 4 after injection with adenovirus encoding LacZ (n = 3, white bar), 3A FoxO1 (n = 3, gray bar), or 3A/LXXAA FoxO1 (n = 3, black bar) were subjected to an overnight fast and killed the next morning for determination of glycogen levels in liver extracts. *Statistically significant difference between 3A and 3A/LXXAA, P < 0.02 by 1-way ANOVA; **statistically significant difference between LacZ and 3A/LXXAA, P < 0.05 by 1-way ANOVA.
Figure 11. Acetylation of transduced FoxO1 in liver from _Lepr_db/db mice.
We sacrificed _Lepr_db/db mice at day 5 after injection of adenovirus encoding 3A (lane 1) or 3A/LXXAA FoxO1 (lane 2) and lysed liver. Liver lysates were immunoprecipitated with anti-Flag monoclonal antibody and Western blotted with the indicated antibodies.
Similar articles
- Role of resveratrol in FOXO1-mediated gluconeogenic gene expression in the liver.
Park JM, Kim TH, Bae JS, Kim MY, Kim KS, Ahn YH. Park JM, et al. Biochem Biophys Res Commun. 2010 Dec 17;403(3-4):329-34. doi: 10.1016/j.bbrc.2010.11.028. Epub 2010 Nov 13. Biochem Biophys Res Commun. 2010. PMID: 21078299 - Nuclear forkhead box O1 controls and integrates key signaling pathways in hepatocytes.
Naïmi M, Gautier N, Chaussade C, Valverde AM, Accili D, Van Obberghen E. Naïmi M, et al. Endocrinology. 2007 May;148(5):2424-34. doi: 10.1210/en.2006-1411. Epub 2007 Feb 15. Endocrinology. 2007. PMID: 17303659 - FoxO-dependent and -independent mechanisms mediate SirT1 effects on IGFBP-1 gene expression.
Gan L, Han Y, Bastianetto S, Dumont Y, Unterman TG, Quirion R. Gan L, et al. Biochem Biophys Res Commun. 2005 Dec 2;337(4):1092-6. doi: 10.1016/j.bbrc.2005.09.169. Epub 2005 Oct 5. Biochem Biophys Res Commun. 2005. PMID: 16236254 - O-GlcNAc modification of FoxO1 increases its transcriptional activity: a role in the glucotoxicity phenomenon?
Kuo M, Zilberfarb V, Gangneux N, Christeff N, Issad T. Kuo M, et al. Biochimie. 2008 May;90(5):679-85. doi: 10.1016/j.biochi.2008.03.005. Epub 2008 Mar 21. Biochimie. 2008. PMID: 18359296 Review. - [Deacetylase SIRT1 and vascular endothelial function].
Wan Z, Yu W, Chen Y, Dai YT. Wan Z, et al. Zhonghua Nan Ke Xue. 2012 Sep;18(9):831-4. Zhonghua Nan Ke Xue. 2012. PMID: 23193674 Review. Chinese.
Cited by
- FoxO transcription factors: their roles in the maintenance of skeletal muscle homeostasis.
Sanchez AM, Candau RB, Bernardi H. Sanchez AM, et al. Cell Mol Life Sci. 2014 May;71(9):1657-71. doi: 10.1007/s00018-013-1513-z. Cell Mol Life Sci. 2014. PMID: 24232446 Free PMC article. Review. - Pck1 gene silencing in the liver improves glycemia control, insulin sensitivity, and dyslipidemia in db/db mice.
Gómez-Valadés AG, Méndez-Lucas A, Vidal-Alabró A, Blasco FX, Chillon M, Bartrons R, Bermúdez J, Perales JC. Gómez-Valadés AG, et al. Diabetes. 2008 Aug;57(8):2199-210. doi: 10.2337/db07-1087. Epub 2008 Apr 28. Diabetes. 2008. PMID: 18443203 Free PMC article. - SIK2 regulates fasting-induced PPARα activity and ketogenesis through p300.
Zhang ZN, Gong L, Lv S, Li J, Tai X, Cao W, Peng B, Qu S, Li W, Zhang C, Luan B. Zhang ZN, et al. Sci Rep. 2016 Mar 17;6:23317. doi: 10.1038/srep23317. Sci Rep. 2016. PMID: 26983400 Free PMC article. - Structures of KIX domain of CBP in complex with two FOXO3a transactivation domains reveal promiscuity and plasticity in coactivator recruitment.
Wang F, Marshall CB, Yamamoto K, Li GY, Gasmi-Seabrook GM, Okada H, Mak TW, Ikura M. Wang F, et al. Proc Natl Acad Sci U S A. 2012 Apr 17;109(16):6078-83. doi: 10.1073/pnas.1119073109. Epub 2012 Apr 2. Proc Natl Acad Sci U S A. 2012. PMID: 22474372 Free PMC article. - Role of SIRT1-FoxO1 signaling in dietary saturated fat-dependent upregulation of liver adiponectin receptor 2 in ethanol-administered mice.
Liang X, Hu M, Rogers CQ, Shen Z, You M. Liang X, et al. Antioxid Redox Signal. 2011 Jul 15;15(2):425-35. doi: 10.1089/ars.2010.3780. Epub 2011 May 4. Antioxid Redox Signal. 2011. PMID: 21194380 Free PMC article.
References
- Tran H., Brunet A., Griffith E.C., Greenberg M.E. The many forks in FOXO’s road. Sci. STKE. 2003;172:RE5.. - PubMed
- Accili D., Arden K.C. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117:421–426.. - PubMed
- Kops G.J., et al. Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature. 1999;398:630–634.. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous