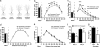Transgenic brain-derived neurotrophic factor expression causes both anxiogenic and antidepressant effects - PubMed (original) (raw)
Transgenic brain-derived neurotrophic factor expression causes both anxiogenic and antidepressant effects
Arvind Govindarajan et al. Proc Natl Acad Sci U S A. 2006.
Abstract
Although neurotrophins have been postulated to have antidepressant properties, their effect on anxiety is not clear. We find that transgenic overexpression of the neurotrophin BDNF has an unexpected facilitatory effect on anxiety-like behavior, concomitant with increased spinogenesis in the basolateral amygdala. Moreover, anxiogenesis and amygdalar spinogenesis are also triggered by chronic stress in control mice but are occluded by BDNF overexpression, thereby suggesting a role for BDNF signaling in stress-induced plasticity in the amygdala. BDNF overexpression also causes antidepressant effects, because transgenic mice exhibit improved performance on the Porsolt forced-swim test and an absence of chronic stress-induced hippocampal atrophy. Thus, structural changes in the amygdala and hippocampus, caused by genetic manipulation of the same molecule BDNF, give rise to contrasting effects on anxiety and depressive symptoms, both of which are major behavioral correlates of stress disorders.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
Fig. 1.
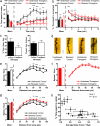
Forebrain BDNF overexpression increases anxiety and spine density in the BLA. (a) CIS causes a decrease in amount of time spent in the center of the open-field apparatus. This is seen both in mean time spent in the center of the apparatus (Left) and when the behavior of the animals is quantified during the entire 10 min of the test (Right). Transgenic mice show the same decrease in time spent in the center of the open field and have no further decrease in time spent in the center when subjected to CIS. (b) CIS causes an increase in locomotor activity, but there is no genotype effect. Furthermore, after 3 min of the test, there is no difference in total movement time between any of the groups, indicating that changes in locomotor activity are not responsible for the differences in anxiety between groups seen in a. (c) Elevated-plus maze data show that transgenic mice spend significantly less time in the open arm compared to control animals (Left). There is no change in activity levels as measured by number of closed-arm entries (Right). (d) Representative photomicrographs of apical dendritic spines from BLA pyramidal neurons of control and transgenic mice, with and without stress. (Scale bar, 10 μm.) (e) In control animals, CIS causes a significant increase in apical spine density, when the mean spine density (Left) along the whole dendrite is considered, or in the distal portion of the dendrite, when segmental analysis is performed (Right). (f) In transgenic animals, there is no significant change in spine density, either in overall mean values (Left) or when segmental analysis is performed (Right). (g) BDNF overexpression leads to the same increase in spine-density as chronic stress. This is shown in both overall mean spine density and segmental analysis (*, P < 0.05; **, P < 0.01; ***, P < 0.001; at the right in a, b, and g, when any statistical significance is noted, it is between the unstressed control group and the least different of the stressed control group and unstressed transgenic group, which are statistically indistinguishable from each other). (h) Correlation analysis shows that mean spine density is strongly correlated with anxiety, as measured by time spent in the center of the open field (R = 0.7, P < 0.01).
Fig. 2.
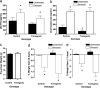
Forebrain BDNF overexpression does not affect commonly used endocrine indicators of stress level in response to CIS. (a and b) Forebrain BDNF overexpression does not affect chronic stress-induced increases in blood plasma concentrations of ACTH (a) and corticosterone (b). (a) In unstressed animals, there is no significant difference between control and transgenic animals in ACTH concentration. Chronically stressed animals have a significant increase in ACTH concentration compared to unstressed animals, but again there is no significant genotype difference. (b) In unstressed animals, there is a significant difference between control and transgenic animals in corticosterone levels. Chronically stressed animals have a significant increase in corticosterone concentration, but there is no genotype effect. (c) There is no difference in body weight between unstressed control and unstressed transgenic mice. (d and e) Forebrain BDNF overexpression does not affect chronic stress-induced weight loss. Unstressed control and transgenic animals show no change in weight, whereas stressed control and transgenic animals show significant weight loss at both 6 days (d) and 11 days (e) after the onset of chronic stress. There is no genotype effect in either unstressed or stressed animals (*, P < 0.05 between unstressed and stressed animals; ‡, P < 0.05 in a comparison between unstressed control and unstressed transgenic animals).
Fig. 3.
Forebrain BDNF overexpression prevents chronic stress-induced dendritic atrophy in the hippocampus and reduces immobility in the Porsolt forced-swim test. (a) Representative _camera lucida_tracings of CA3 pyramidal neurons from control and BDNF-overexpressing transgenic mice with and without stress. (Scale bar, 50 μm.) (b and c) Effects of CIS on CA3 apical dendritic morphology in control mice. (Left) Mean values for dendritic length (b) and number of branch points (c) in a 50-μm shell as a function of the radial distance from the soma. (Right) These mean values are computed by averaging across incremental steps of 50 μm along the apical dendrites using Sholl’s analysis. (*, P < 0.05; **, P < 0.01; ***, P < 0.001). (d and e) Effects of CIS on CA3 apical dendritic morphology in transgenic mice. (Left) Mean values for dendritic length (d) and number of branch points (e) in a 50-μm shell as a function of the radial distance from the soma. (Right) These mean values are computed by averaging across incremental steps of 50 μm along the apical dendrites using Sholl’s analysis. (f) Forebrain BDNF overexpression improves performance on the Porsolt forced-swim test. On both day 1 and day 2, the transgenic animals show less immobility as compared to control littermates (*, P < 0.05 between control and transgenic animals for each day; †, P < 0.01 between days 1 and 2 for control animals; ‡, P < 0.01 between days 1 and 2 for transgenic animals). (g) Forebrain BDNF overexpression improves performance in the Porsolt forced-swim test when the difference in immobility between days 2 and 1 is considered. CTL, control; TG, transgenic (*, P < 0.05).
Similar articles
- Effects of antidepressant treatment on mice lacking brain-derived neurotrophic factor expression through promoter IV.
Sakata K, Mastin JR, Duke SM, Vail MG, Overacre AE, Dong BE, Jha S. Sakata K, et al. Eur J Neurosci. 2013 Jun;37(11):1863-74. doi: 10.1111/ejn.12148. Epub 2013 Feb 13. Eur J Neurosci. 2013. PMID: 23406189 - Behavioral and serotonergic consequences of decreasing or increasing hippocampus brain-derived neurotrophic factor protein levels in mice.
Deltheil T, Guiard BP, Cerdan J, David DJ, Tanaka KF, Repérant C, Guilloux JP, Coudoré F, Hen R, Gardier AM. Deltheil T, et al. Neuropharmacology. 2008 Nov;55(6):1006-14. doi: 10.1016/j.neuropharm.2008.08.001. Epub 2008 Aug 12. Neuropharmacology. 2008. PMID: 18761360 Review. - Tianeptine increases brain-derived neurotrophic factor expression in the rat amygdala.
Reagan LP, Hendry RM, Reznikov LR, Piroli GG, Wood GE, McEwen BS, Grillo CA. Reagan LP, et al. Eur J Pharmacol. 2007 Jun 22;565(1-3):68-75. doi: 10.1016/j.ejphar.2007.02.023. Epub 2007 Feb 20. Eur J Pharmacol. 2007. PMID: 17368617 - Strain-dependent variations in visceral sensitivity: relationship to stress, anxiety and spinal glutamate transporter expression.
Moloney RD, Dinan TG, Cryan JF. Moloney RD, et al. Genes Brain Behav. 2015 Apr;14(4):319-29. doi: 10.1111/gbb.12216. Epub 2015 Apr 21. Genes Brain Behav. 2015. PMID: 25851919
Cited by
- Research Progress on NMDA Receptor Enhancement Drugs for the Treatment of Depressive Disorder.
Liu R, Liu N, Ma L, Liu Y, Huang Z, Peng X, Zhuang C, Niu J, Yu J, Du J. Liu R, et al. CNS Drugs. 2024 Dec;38(12):985-1002. doi: 10.1007/s40263-024-01123-x. Epub 2024 Oct 8. CNS Drugs. 2024. PMID: 39379772 Review. - Brain RFamide Neuropeptides in Stress-Related Psychopathologies.
Kovács A, Szabó E, László K, Kertes E, Zagorácz O, Mintál K, Tóth A, Gálosi R, Berta B, Lénárd L, Hormay E, László B, Zelena D, Tóth ZE. Kovács A, et al. Cells. 2024 Jun 25;13(13):1097. doi: 10.3390/cells13131097. Cells. 2024. PMID: 38994950 Free PMC article. Review. - Precision Medicine in Antidepressants Treatment.
Tsermpini EE, Serretti A, Dolžan V. Tsermpini EE, et al. Handb Exp Pharmacol. 2023;280:131-186. doi: 10.1007/164_2023_654. Handb Exp Pharmacol. 2023. PMID: 37195310 Review. - Biological effects of exposure to 2650 MHz electromagnetic radiation on the behavior, learning, and memory of mice.
Zheng R, Zhang X, Gao Y, Gao D, Gong W, Zhang C, Dong G, Li Z. Zheng R, et al. Brain Behav. 2023 Jun;13(6):e3004. doi: 10.1002/brb3.3004. Epub 2023 Apr 28. Brain Behav. 2023. PMID: 37118929 Free PMC article.
References
- McEwen B. S., Sapolsky R. M. Curr. Opin. Neurobiol. 1995;5:205–216. - PubMed
- McEwen B. S. Annu. Rev. Neurosci. 1999;22:105–122. - PubMed
- Sapolsky R. M. Nat. Neurosci. 2002;5:1111–1113. - PubMed
- Bremner J. D. Curr. Psychiatry Rep. 2002;4:254–263. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases