Modulation of beta-amyloid precursor protein trafficking and processing by the low density lipoprotein receptor family - PubMed (original) (raw)
Modulation of beta-amyloid precursor protein trafficking and processing by the low density lipoprotein receptor family
Judy A Cam et al. Mol Neurodegener. 2006.
Abstract
Amyloid-beta peptide (Abeta) accumulation in the brain is an early, toxic event in the pathogenesis of Alzheimer's disease (AD). Abeta is produced by proteolytic processing of a transmembrane protein, beta-amyloid precursor protein (APP), by beta- and gamma-secretases. Mounting evidence has demonstrated that alterations in APP cellular trafficking and localization directly impact its processing to Abeta. Recent studies have shown that members of the low-density lipoprotein receptor family, including LRP, LRP1B, SorLA/LR11, and apolipoprotein E (apoE) receptor 2, interact with APP and regulate its endocytic trafficking. Another common feature of these receptors is their ability to bind apoE, which exists in three isoforms in humans and the presence of the epsilon4 allele represents a genetic risk factor for AD. In this review, we summarize the current understanding of the function of these apoE receptors with a focus on their role in APP trafficking and processing. Knowledge of the interactions between these distinct low-density lipoprotein receptor family members and APP may ultimately influence future therapies for AD.
Figures
Figure 1
Schematic representation of APP processing. APP is a type I transmembrane protein that can undergo two separate proteolytic pathways. In the non-amyloidogenic pathway, APP is processed by an α-secretase that clips within the Aβ region (thus precluding its formation), resulting in the release of a soluble ~110–120 kDa N-terminal APP fragment (sAPPα). This pathway also releases a CTF that is 83 amino acids in length (C83). C83 can also be cleaved by a γ-secretase to release a small, non-toxic 3 kDa fragment known as p3 and a γCTF known as APP intracellular domain (AICD). In the amyloidogenic pathway, APP is cleaved first by β-secretase releasing a sAPPβ fragment and leaving a 99 amino acid CTF attached to the membrane (C99). C99 is subsequently cleaved by a γ-secretase, within its intramembrane region to release the Aβ peptide and AICD. SP, Signal Peptide; KPI, Kunitz-type Proteinase Inhibitor domain.
Figure 2
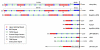
Schematic representation of members of the LDLR family. Members of the LDLR family have diverse functions from cholesterol metabolism, Reelin and Wnt signalling, to intracellular transport. Despite multiple functions, they share common structural motifs, including ligand-binding repeats, epidermal growth factor (EGF) repeats, YWTD spacer domains, a single transmembrane domain and a short cytoplasmic domain containing conserved endocytic motifs. FN, fibronectin.
Figure 3
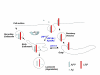
Model of APP processing pathways mediated by LDL receptor family members. LRP endocytosis enhances APP endocytosis and processing to Aβ. Due to its slow rate of endocytosis LRP1B retains APP at the cell surface and decreases its processing to Aβ. ApoER2 enhances interactions between APP and F-spondin at the cell surface and also decreases its processing to Aβ. SorLA/LR11 may shuttle APP to the Golgi and prevent its processing by β-secretase in the early endosome, thus decreasing processing to Aβ.
Similar articles
- Lipoprotein receptors and cholesterol in APP trafficking and proteolytic processing, implications for Alzheimer's disease.
Marzolo MP, Bu G. Marzolo MP, et al. Semin Cell Dev Biol. 2009 Apr;20(2):191-200. doi: 10.1016/j.semcdb.2008.10.005. Epub 2008 Oct 17. Semin Cell Dev Biol. 2009. PMID: 19041409 Free PMC article. Review. - ApoE-isoform-dependent cellular uptake of amyloid-β is mediated by lipoprotein receptor LR11/SorLA.
Yajima R, Tokutake T, Koyama A, Kasuga K, Tezuka T, Nishizawa M, Ikeuchi T. Yajima R, et al. Biochem Biophys Res Commun. 2015 Jan 2;456(1):482-8. doi: 10.1016/j.bbrc.2014.11.111. Epub 2014 Dec 5. Biochem Biophys Res Commun. 2015. PMID: 25482438 - Functional role of lipoprotein receptors in Alzheimer's disease.
Jaeger S, Pietrzik CU. Jaeger S, et al. Curr Alzheimer Res. 2008 Feb;5(1):15-25. doi: 10.2174/156720508783884675. Curr Alzheimer Res. 2008. PMID: 18288927 Review. - LRP in amyloid-beta production and metabolism.
Bu G, Cam J, Zerbinatti C. Bu G, et al. Ann N Y Acad Sci. 2006 Nov;1086:35-53. doi: 10.1196/annals.1377.005. Ann N Y Acad Sci. 2006. PMID: 17185504 Review. - Trafficking in Alzheimer's Disease: Modulation of APP Transport and Processing by the Transmembrane Proteins LRP1, SorLA, SorCS1c, Sortilin, and Calsyntenin.
Eggert S, Thomas C, Kins S, Hermey G. Eggert S, et al. Mol Neurobiol. 2018 Jul;55(7):5809-5829. doi: 10.1007/s12035-017-0806-x. Epub 2017 Oct 27. Mol Neurobiol. 2018. PMID: 29079999 Review.
Cited by
- Apolipoprotein E and Alzheimer's disease: molecular mechanisms and therapeutic opportunities.
Cedazo-Mínguez A. Cedazo-Mínguez A. J Cell Mol Med. 2007 Nov-Dec;11(6):1227-38. doi: 10.1111/j.1582-4934.2007.00130.x. J Cell Mol Med. 2007. PMID: 18205697 Free PMC article. Review. - Use of Caenorhabditis elegans as a model to study Alzheimer's disease and other neurodegenerative diseases.
Alexander AG, Marfil V, Li C. Alexander AG, et al. Front Genet. 2014 Sep 5;5:279. doi: 10.3389/fgene.2014.00279. eCollection 2014. Front Genet. 2014. PMID: 25250042 Free PMC article. Review. - Brain traffic: subcellular transport of the amyloid precursor protein.
Mayeux R, St George-Hyslop P. Mayeux R, et al. Arch Neurol. 2009 Apr;66(4):433-4. doi: 10.1001/archneurol.2009.29. Arch Neurol. 2009. PMID: 19364927 Free PMC article. No abstract available. - Age-Related Decline in Brain and Hepatic Clearance of Amyloid-Beta is Rectified by the Cholinesterase Inhibitors Donepezil and Rivastigmine in Rats.
Mohamed LA, Qosa H, Kaddoumi A. Mohamed LA, et al. ACS Chem Neurosci. 2015 May 20;6(5):725-36. doi: 10.1021/acschemneuro.5b00040. Epub 2015 Mar 30. ACS Chem Neurosci. 2015. PMID: 25782004 Free PMC article. - Apolipoprotein E, amyloid-beta, and neuroinflammation in Alzheimer's disease.
Dorey E, Chang N, Liu QY, Yang Z, Zhang W. Dorey E, et al. Neurosci Bull. 2014 Apr;30(2):317-30. doi: 10.1007/s12264-013-1422-z. Epub 2014 Mar 20. Neurosci Bull. 2014. PMID: 24652457 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous