Uncoiling mechanics of Escherichia coli type I fimbriae are optimized for catch bonds - PubMed (original) (raw)
Comparative Study
Uncoiling mechanics of Escherichia coli type I fimbriae are optimized for catch bonds
Manu Forero et al. PLoS Biol. 2006 Sep.
Abstract
We determined whether the molecular structures through which force is applied to receptor-ligand pairs are tuned to optimize cell adhesion under flow. The adhesive tethers of our model system, Escherichia coli, are type I fimbriae, which are anchored to the outer membrane of most E. coli strains. They consist of a fimbrial rod (0.3-1.5 microm in length) built from a helically coiled structural subunit, FimA, and an adhesive subunit, FimH, incorporated at the fimbrial tip. Previously reported data suggest that FimH binds to mannosylated ligands on the surfaces of host cells via catch bonds that are enhanced by the shear-originated tensile force. To understand whether the mechanical properties of the fimbrial rod regulate the stability of the FimH-mannose bond, we pulled the fimbriae via a mannosylated tip of an atomic force microscope. Individual fimbriae rapidly elongate for up to 10 microm at forces above 60 pN and rapidly contract again at forces below 25 pN. At intermediate forces, fimbriae change length more slowly, and discrete 5.0 +/- 0.3-nm changes in length can be observed, consistent with uncoiling and coiling of the helical quaternary structure of one FimA subunit at a time. The force range at which fimbriae are relatively stable in length is the same as the optimal force range at which FimH-mannose bonds are longest lived. Higher or lower forces, which cause shorter bond lifetimes, cause rapid length changes in the fimbria that help maintain force at the optimal range for sustaining the FimH-mannose interaction. The modulation of force and the rate at which it is transmitted from the bacterial cell to the adhesive catch bond present a novel physiological role for the fimbrial rod in bacterial host cell adhesion. This suggests that the mechanical properties of the fimbrial shaft have codeveloped to optimize the stability of the terminal adhesive under flow.
Conflict of interest statement
Competing interests. The authors have declared that no competing interests exist.
Figures
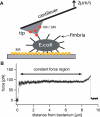
Figure 1. Force Measurements on Type I Fimbriae of E. coli
(A) Experimental setup. The tip (gray) of an AFM cantilever is coated with 1M-BSA (red) or 3M-RNAseB (yellow). An E. coli bacterium is attached to a 3M-covered glass surface. After the bacterium is approached with the AFM tip, one or multiple fimbriae can bind. The cantilever base is then pulled away from the bacterium at a constant velocity. (B) Force/extension curve for a single fimbria measured at a constant velocity of 2 μm/s. When pulled at various constant velocities, fimbriae extend to multiple times their original length of ~0.7 μm. The force profile shows three distinct regions: first, the force increases quickly with separation. Second, this is followed by a constant-force region where the AFM tip can be pulled away for several micrometers. Third, the force then increases nonlinearly before the bond between the mannose coated AFM tip and the fimbria finally breaks. (The spring constant of the cantilever used in this pull was 7.9 pN/nm.)
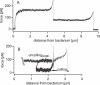
Figure 2. Force/Extension Curves of Fimbriae
(A) Force/extension curve when two fimbriae attach to a mannose-coated tip retracting at 2 μm/s. After the first fimbria snaps off the tip, the second fimbria continues to be attached and is pulled until it detaches. The pulling force in the first constant-force region is close to twice that in the second constant-force region, and two distinct break events are observed, indicating that individual fimbriae are being probed. (B) Reversible uncoiling and coiling of a single fimbria. After pulling a fimbria for a set distance, the pulling direction was reversed and the fimbria was observed to coil back at a nonzero force. After a second reversal to the initial pulling velocity, the force returned to its original level. These data show that both uncoiling and coiling are sequential and reversible. The spring constant of the cantilever used in these pulls was 7.9 pN/nm, and the pulls were done on two different bacteria. Most of the >5,000 successful constant velocity pulls we observed are qualitatively similar to those in Figures 1B or 2A with two (shown here) or more fimbriae. The main difference was in the length of the constant-force region as expected from the variability of the length of fimbriae.
Figure 3. Discrete Coiling and Uncoiling Events of a Single Fimbria
(A) Cartoon showing the structure of fimbriae attached to an AFM tip. The lectin domain of FimH binds specifically to mannose on the AFM tip. The fimbrial tip consists of the FimH adhesin (lectin plus pilin domain) and the FimG and FimF monomers. Below them is the 7-nm-diameter shaft (also called pilus, fimbria) made from helically coiled FimA subunits. FimA subunits contribute 0.7 nm to the length of the helical shaft [9]. By homology to the pilin domain of FimH, the length of the FimA subunit is approximately 5.7 nm. The steps measured in (B) are in good agreement with the 5-nm extension expected from (A). (B) Discrete elongation and contraction events are seen when a single fimbria is pulled at constant force just below and above the force where coiling and uncoiling rates have equal probability (f bal). The bottom trace shows that the force is temporarily lowered whenever a length increase is observed. The histogram shows the frequency of both coiling and uncoiling events combined with a peak at 5.0 ± 0.3 nm. The frequency of these single-step events increases when the applied force approaches f bal, as expected from Equation 3. The spring constant of the cantilever used in this experiment was 6.5 pN/nm. The individual events in the histogram were obtained from four different bacteria.
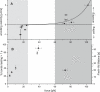
Figure 4. Comparison between the Uncoiling/Coiling Forces of the Fimbrial Shaft versus the Lifetime of the FimH–Mannose Bond
(A) Uncoiling force of fimbriae as a function of pulling velocity (solid squares). Three regimes can be discerned: fimbriae uncoil significantly at forces greater than 60 pN (dark gray); they also coil back at significant velocities generating forces up to 25 pN (light gray); between 25 and 60 pN, fimbriae do not change significantly in length. A two-state model for uncoiling, assuming the coiled and uncoiled states are separated by a single energy barrier, was fit and is shown as a solid line. The model fits the data well and yields a height of the uncoiling and coiling energy barriers of 29_k_ b T and 4_k_ b T, respectively. The spring constant of the AFM cantilever for this experiment was of 6.7 pN/nm, and the data are taken from one bacterium (n > 8 data points for each point). Other bacteria probed showed the same behavior. Similar curves were also obtained by keeping the force constant and measuring the velocity. One example is shown in Figure S1. (B) Effect of force on FimH–1M bond lifetimes. The solid diamonds (right axis) show the mean lifetimes of fimbriated FimH–1M bonds measured with the AFM by holding a constant force on fimbriae and waiting for the bonds to break. The decrease of the lifetime as force increases from 40 to 60 pN shows the weakening of the bond at high forces. The solid triangles (left axis) show the fraction of FimH–1M bonds that last longer than 1 s in flow chamber experiments (n = 4 to 6 for each point). These experiments are described in detail in Figure 5. This fraction increases from 20% to 80% between 2.5 and 38 pN, demonstrating the catch-bond nature of FimH–1M bonds. Error bars are standard error of the mean.
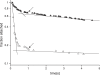
Figure 5. FimH–1M Bond Lifetimes in a Flow Chamber
The binding of 1M beads to fimbriae-coated surfaces was monitored in flow chambers at two well-defined flow rates that create drag forces of 2.5 pN (open triangles) and 37 pN (solid triangles) on the beads. Beads moving in the field of view alternately paused (bound to the surface in a stationary manner for a period of time) and moved at the hydrodynamic velocity of the fluid flow. The lifetime of the pauses was measured and assumed to correspond to single-bond lifetimes. The lifetime of these pauses is plotted here as the fraction of all pauses that survive to the time indicated on the _x_-axis. There are two distinct decay rates under each condition, suggesting a weak and a strong conformational state, each corresponding to short-lived and long-lived binding, respectively. The fraction of pauses lasting more than 1 s (arrows) was calculated directly from raw data like these and used in Figure 4B. This fraction increased with force, consistent with the notion that FimH forms a catch bond with mannose because of a force-induced switch to a long-lived state. The lines correspond to the slopes of the exponential fits of the lifetimes in the first 500 ms or between 2 and 20 s, as described in the Materials and Methods section.
Comment in
- Bacterial fimbriae designed to stay with the flow.
Gross L. Gross L. PLoS Biol. 2006 Sep;4(9):e314. doi: 10.1371/journal.pbio.0040314. Epub 2006 Aug 29. PLoS Biol. 2006. PMID: 20076642 Free PMC article. No abstract available.
Similar articles
- Dynamic modulation of fimbrial extension and FimH-mannose binding force on live bacteria under pH changes: a molecular atomic force microscopy analysis.
Jacquot A, Sakamoto C, Razafitianamaharavo A, Caillet C, Merlin J, Fahs A, Ghigo JM, Beloin C, Duval JF, Francius G. Jacquot A, et al. J Biomed Nanotechnol. 2014 Nov;10(11):3361-72. doi: 10.1166/jbn.2014.1905. J Biomed Nanotechnol. 2014. PMID: 26000394 - Catch-bond mechanism of the bacterial adhesin FimH.
Sauer MM, Jakob RP, Eras J, Baday S, Eriş D, Navarra G, Bernèche S, Ernst B, Maier T, Glockshuber R. Sauer MM, et al. Nat Commun. 2016 Mar 7;7:10738. doi: 10.1038/ncomms10738. Nat Commun. 2016. PMID: 26948702 Free PMC article. - The bacterial fimbrial tip acts as a mechanical force sensor.
Aprikian P, Interlandi G, Kidd BA, Le Trong I, Tchesnokova V, Yakovenko O, Whitfield MJ, Bullitt E, Stenkamp RE, Thomas WE, Sokurenko EV. Aprikian P, et al. PLoS Biol. 2011 May;9(5):e1000617. doi: 10.1371/journal.pbio.1000617. Epub 2011 May 10. PLoS Biol. 2011. PMID: 21572990 Free PMC article. - Catch-bond mechanism of force-enhanced adhesion: counterintuitive, elusive, but ... widespread?
Sokurenko EV, Vogel V, Thomas WE. Sokurenko EV, et al. Cell Host Microbe. 2008 Oct 16;4(4):314-23. doi: 10.1016/j.chom.2008.09.005. Cell Host Microbe. 2008. PMID: 18854236 Free PMC article. Review. - Molecular structure of adhesin domains in Escherichia coli fimbriae.
Westerlund-Wikström B, Korhonen TK. Westerlund-Wikström B, et al. Int J Med Microbiol. 2005 Oct;295(6-7):479-86. doi: 10.1016/j.ijmm.2005.06.010. Int J Med Microbiol. 2005. PMID: 16238022 Review.
Cited by
- Surface organelles assembled by secretion systems of Gram-negative bacteria: diversity in structure and function.
Thanassi DG, Bliska JB, Christie PJ. Thanassi DG, et al. FEMS Microbiol Rev. 2012 Nov;36(6):1046-82. doi: 10.1111/j.1574-6976.2012.00342.x. Epub 2012 May 24. FEMS Microbiol Rev. 2012. PMID: 22545799 Free PMC article. Review. - The shaft of the type 1 fimbriae regulates an external force to match the FimH catch bond.
Zakrisson J, Wiklund K, Axner O, Andersson M. Zakrisson J, et al. Biophys J. 2013 May 21;104(10):2137-48. doi: 10.1016/j.bpj.2013.03.059. Biophys J. 2013. PMID: 23708354 Free PMC article. - The biomechanical properties of E. coli pili for urinary tract attachment reflect the host environment.
Andersson M, Uhlin BE, Fällman E. Andersson M, et al. Biophys J. 2007 Nov 1;93(9):3008-14. doi: 10.1529/biophysj.107.110643. Epub 2007 Aug 3. Biophys J. 2007. PMID: 17675342 Free PMC article. - Tension can directly suppress Aurora B kinase-triggered release of kinetochore-microtubule attachments.
de Regt AK, Clark CJ, Asbury CL, Biggins S. de Regt AK, et al. Nat Commun. 2022 Apr 20;13(1):2152. doi: 10.1038/s41467-022-29542-8. Nat Commun. 2022. PMID: 35443757 Free PMC article. - Mechanics of biofilms formed of bacteria with fimbriae appendages.
Jin X, Marshall JS. Jin X, et al. PLoS One. 2020 Dec 8;15(12):e0243280. doi: 10.1371/journal.pone.0243280. eCollection 2020. PLoS One. 2020. PMID: 33290393 Free PMC article.
References
- Thomas WE, Trintchina E, Forero M, Vogel V, Sokurenko EV. Bacterial adhesion to target cells enhanced by shear force. Cell. 2002;109:913–923. - PubMed
- Thomas WE, Nilsson LM, Forero M, Sokurenko EV, Vogel V. Shear-dependent 'stick-and-roll' adhesion of type 1 fimbriated Escherichia coli . Mol Microbiol. 2004;53:1545–1557. - PubMed
- Forero M, Thomas WE, Bland C, Nilsson LM, Sokurenko EV, et al. A catch-bond based nanoadhesive sensitive to shear stress. Nano Lett. 2004;4:1593–1597.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources