A mitotically inheritable unit containing a MAP kinase module - PubMed (original) (raw)
Comparative Study
. 2006 Sep 5;103(36):13445-50.
doi: 10.1073/pnas.0603693103. Epub 2006 Aug 24.
Affiliations
- PMID: 16938837
- PMCID: PMC1569183
- DOI: 10.1073/pnas.0603693103
Comparative Study
A mitotically inheritable unit containing a MAP kinase module
Sébastien Kicka et al. Proc Natl Acad Sci U S A. 2006.
Abstract
Prions are novel kinds of hereditary units, relying solely on proteins, that are infectious and inherited in a non-Mendelian fashion. To date, they are either based on autocatalytic modification of a 3D conformation or on autocatalytic cleavage. Here, we provide further evidence that in the filamentous fungus Podospora anserina, a MAP kinase cascade is probably able to self-activate and generate C, a hereditary unit that bears many similarities to prions and triggers cell degeneration. We show that in addition to the MAPKKK gene, both the MAPKK and MAPK genes are necessary for the propagation of C, and that overexpression of MAPK as that of MAPKKK facilitates the appearance of C. We also show that a correlation exists between the presence of C and localization of the MAPK inside nuclei. These data emphasize the resemblance between prions and a self-positively regulated cascade in terms of their transmission. This thus further expands the concept of protein-base inheritance to regulatory networks that have the ability to self-activate.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
Fig. 1.
PaMKK1 and PaASK1 act upstream of PaMpk1. Protein extracts (20 μg) from a wild-type strain grown for different durations (24, 36, and 48 h) on M2 medium and from the Δ_PaMpk1_, IDC404, and IDC118 mutants grown for 48 h were separated on gels and probed with antibodies that specifically detect the unphosphorylated form (anti-MAPK) and the phosphorylated form (anti-phospho-MAPK) of PaMpk1.
Fig. 2.
Overexpression of PaMpk1 enhances CG. (A) Quantification of the overexpression of PaMpk1-HA in the strains indicated. (Upper) Western blotting with anti-HA antibodies. (Lower) Coomassie staining of a gel with the same samples. The fold overexpression compared with wild-type (WT) has been evaluated on four different blots, of which the one presented in the figure is revealed by nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate. In the three others, the HA-tag was detected with ECL. The values obtained after normalization with the wild-type strain were 1.7 ± 0.3 for surPaMpk1-HA5, 1.5 ± 0.1 for surPaMpk1HA7, 1.7 ± 0.5 for surPaMpk1-HA5 surPaMpk1HA7, 1.4 ± 0.5 for surPaMpk1-HA1, and 3.75 ± 1.0 for surPaMpk1-HA1 surPaMpk1-HA5. CG, ability of the strain to trigger CG. (B) Experimental setup to detect CG and resulting plates. CG is visible in the surPaMpk1-HA1 + surPaMpk1-HA5 overexpressing strain yielding darkly pigmented thalli and is less pronounced in the surPaMpk1-HA1 strain and in the surPaMpk1-HA5 + surPaMpk1-HA7 strain (not shown).
Fig. 3.
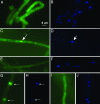
Localization of PaMpk1-GFP. (A and B) Diffuse localization of PaMpk1-GFP in growing hyphae of a wild-type strain carrying the MGFP4 transgene. (C and D) Typical PaMpk1-GFP nuclear localization in 3-day-old hyphae of a wild-type strain with the MGFP4 transgene. (E and F) Lack of PaMpk1-GFP nuclear localization in 3-day-old hyphae in strains carrying the IDC404 mutation and the MGFP4 transgene. The same result is seen in strains with the IDC118 mutation carrying the MGFP4 transgene. (G and H) Nuclear localization of PaMpk1-GFP in growing hyphae of a _CG AS6_-5 culture carrying the MGFP4 transgene. (I and J) Diffuse localization of PaMpk1-GFP in growing hyphae of an _NG AS6_-5 culture carrying the MGFP4 transgene. A, C, E, G, and I: GFP staining to show PaMpk1-GFP. B, D, F, H, and J: DAPI staining to show nuclei. Arrows point toward nuclei containing PaMpk1.
Fig. 4.
Effect of the PaNox1 mutation on PaMpk1. (A) Protein extract of the IDC343 mutant analyzed as in Fig. 1 shows that PaMpk1 is phosphorylated in a strain lacking PaNox1. (B) Lack of nuclear localization of PaMpk1 in 3-day-old hyphae.
Similar articles
- IDC1, a pezizomycotina-specific gene that belongs to the PaMpk1 MAP kinase transduction cascade of the filamentous fungus Podospora anserina.
Jamet-Vierny C, Debuchy R, Prigent M, Silar P. Jamet-Vierny C, et al. Fungal Genet Biol. 2007 Dec;44(12):1219-30. doi: 10.1016/j.fgb.2007.04.005. Epub 2007 Apr 19. Fungal Genet Biol. 2007. PMID: 17517525 - Genetic control of an epigenetic cell degeneration syndrome in Podospora anserina.
Haedens V, Malagnac F, Silar P. Haedens V, et al. Fungal Genet Biol. 2005 Jun;42(6):564-77. doi: 10.1016/j.fgb.2005.03.011. Fungal Genet Biol. 2005. PMID: 15869888 - The MpkA MAP kinase module regulates cell wall integrity signaling and pyomelanin formation in Aspergillus fumigatus.
Valiante V, Jain R, Heinekamp T, Brakhage AA. Valiante V, et al. Fungal Genet Biol. 2009 Dec;46(12):909-18. doi: 10.1016/j.fgb.2009.08.005. Epub 2009 Aug 26. Fungal Genet Biol. 2009. PMID: 19715768 - Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases.
Owens DM, Keyse SM. Owens DM, et al. Oncogene. 2007 May 14;26(22):3203-13. doi: 10.1038/sj.onc.1210412. Oncogene. 2007. PMID: 17496916 Review. - Cell death by incompatibility in the fungus Podospora.
Pinan-Lucarré B, Paoletti M, Clavé C. Pinan-Lucarré B, et al. Semin Cancer Biol. 2007 Apr;17(2):101-11. doi: 10.1016/j.semcancer.2006.11.009. Epub 2006 Dec 15. Semin Cancer Biol. 2007. PMID: 17204431 Review.
Cited by
- The relationship of prions and translation.
Wickner RB, Edskes HK, Shewmaker FP, Kryndushkin D, Nemecek J, McGlinchey R, Bateman D. Wickner RB, et al. Wiley Interdiscip Rev RNA. 2010 Jul-Aug;1(1):81-9. doi: 10.1002/wrna.8. Wiley Interdiscip Rev RNA. 2010. PMID: 21339834 Free PMC article. Review. - The crucial role of the Pls1 tetraspanin during ascospore germination in Podospora anserina provides an example of the convergent evolution of morphogenetic processes in fungal plant pathogens and saprobes.
Lambou K, Malagnac F, Barbisan C, Tharreau D, Lebrun MH, Silar P. Lambou K, et al. Eukaryot Cell. 2008 Oct;7(10):1809-18. doi: 10.1128/EC.00149-08. Epub 2008 Aug 29. Eukaryot Cell. 2008. PMID: 18757568 Free PMC article. - The NADPH oxidase-mediated production of hydrogen peroxide (H(2)O(2)) and resistance to oxidative stress in the necrotrophic pathogen Alternaria alternata of citrus.
Yang SL, Chung KR. Yang SL, et al. Mol Plant Pathol. 2012 Oct;13(8):900-14. doi: 10.1111/j.1364-3703.2012.00799.x. Epub 2012 Mar 21. Mol Plant Pathol. 2012. PMID: 22435666 Free PMC article. - A RID-like putative cytosine methyltransferase homologue controls sexual development in the fungus Podospora anserina.
Grognet P, Timpano H, Carlier F, Aït-Benkhali J, Berteaux-Lecellier V, Debuchy R, Bidard F, Malagnac F. Grognet P, et al. PLoS Genet. 2019 Aug 14;15(8):e1008086. doi: 10.1371/journal.pgen.1008086. eCollection 2019 Aug. PLoS Genet. 2019. PMID: 31412020 Free PMC article.
References
- Wickner R. B., Edskes H. K., Roberts B. T., Baxa U., Pierce M. M., Ross E. D., Brachmann A. Genes Dev. 2004;18:470–485. - PubMed
- Haedens V., Malagnac F., Silar P. Fungal Genet. Biol. 2005;42:564–577. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources