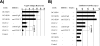DC-SIGN and CLEC-2 mediate human immunodeficiency virus type 1 capture by platelets - PubMed (original) (raw)
. 2006 Sep;80(18):8951-60.
doi: 10.1128/JVI.00136-06.
Elizabeth J Soilleux, Peter Simpson, Heike Hofmann, Thomas Gramberg, Andrea Marzi, Martina Geier, Elizabeth A Stewart, Jutta Eisemann, Alexander Steinkasserer, Katsue Suzuki-Inoue, Gemma L Fuller, Andrew C Pearce, Steve P Watson, James A Hoxie, Frederic Baribaud, Stefan Pöhlmann
Affiliations
- PMID: 16940507
- PMCID: PMC1563896
- DOI: 10.1128/JVI.00136-06
DC-SIGN and CLEC-2 mediate human immunodeficiency virus type 1 capture by platelets
Chawaree Chaipan et al. J Virol. 2006 Sep.
Abstract
Platelets can engulf human immunodeficiency virus type 1 (HIV-1), and a significant amount of HIV-1 in the blood of infected individuals is associated with these cells. However, it is unclear how platelets capture HIV-1 and whether platelet-associated virus remains infectious. DC-SIGN and other lectins contribute to capture of HIV-1 by dendritic cells (DCs) and facilitate HIV-1 spread in DC/T-cell cocultures. Here, we show that platelets express both the C-type lectin-like receptor 2 (CLEC-2) and low levels of DC-SIGN. CLEC-2 bound to HIV-1, irrespective of the presence of the viral envelope protein, and facilitated HIV-1 capture by platelets. However, a substantial fraction of the HIV-1 binding activity of platelets was dependent on DC-SIGN. A combination of DC-SIGN and CLEC-2 inhibitors strongly reduced HIV-1 association with platelets, indicating that these lectins are required for efficient HIV-1 binding to platelets. Captured HIV-1 was maintained in an infectious state over several days, suggesting that HIV-1 can escape degradation by platelets and might use these cells to promote its spread. Our results identify CLEC-2 as a novel HIV-1 attachment factor and provide evidence that platelets capture and transfer infectious HIV-1 via DC-SIGN and CLEC-2, thereby possibly facilitating HIV-1 dissemination in infected patients.
Figures
FIG. 1.
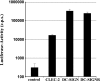
CLEC-2 facilitates HIV-1 transmission by 293T cells. The indicated lectins and a control vector were transiently expressed in 293T cells, and the cells were incubated with NL4-3 Luc, washed, and cocultivated with CEMx174 R5 target cells. Luciferase activities in cell lysates were determined 3 days after cocultivation. A representative experiment performed in triplicate is shown, and error bars indicate standard deviations. Similar results were obtained in two independent experiments. c.p.s., counts per second.
FIG. 2.
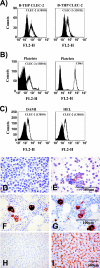
Platelets and megakaryocytes express CLEC-2. (A) B-THP CLEC-2 cells were stained with anti-CLEC-2 antibodies (white) or isotype-matched control antibodies (black) and analyzed by flow cytometry. Similar results were obtained in three independent experiments. (B) Flow cytometric analyses of CLEC-2 (white, left panel) and CD61 (white, right panel) expression on platelets. Staining with isotype-matched control antibodies is shown in black. The results were confirmed in five independent experiments with platelet preparations from different donors. Identical results were obtained upon staining with antibody 13H11. (C) The megakaryocytic cell lines DAMI and HEL were stained with anti-CLEC-2 (white) or isotype-matched control antibody (black) and analyzed by flow cytometry. Similar results were obtained in two independent experiments. FL2-H, fluorescence in channel 2-height. (D to I) Sections of human tissue or B-THP CLEC-2 cells were formalin fixed, paraffin embedded, and immunostained (brown) for CLEC-2 or CD61 or immunostained with omission of the primary antibody as a control. B-THP CLEC-2 cells were control stained (D) or stained with CLEC-2-specific antiserum (E). Bone marrow was stained for CD61 (F) or CLEC-2 (G). Liver sections were control stained (H) or stained for CLEC-2 (I).
FIG. 3.
CLEC-2 binds HIV-1, and bound virus is infectious for adjacent target cells. (A) B-THP cell lines were incubated with the indicated inhibitors and pulsed with p24-normalized HIV-1 NL4-3 Luc. Unbound virus was removed, cells were lysed, and the amount of p24 antigen in lysates was determined. The amount of virus recovered is expressed as a percentage of the input virus. A representative experiment is shown. Similar results were obtained in three independent experiments. (B) The experiment was conducted as described for panel A. However, after removal of unbound virus, platelets were cocultivated with CEMx174 R5 target cells (+) and luciferase activities in cell lysates determined 3 days after cocultivation. A representative experiment is shown. Results were confirmed in three independent experiments. Error bars represent standard deviations. P values were determined using a two-sided dependent-sample t test. IgG, immunoglobulin G; c.p.s., counts per second.
FIG. 4.
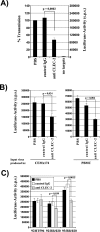
Specificity of the HIV-1 interaction with CLEC-2. The indicated B-THP cell lines were incubated with p24-normalized HIV-1 NL4-3 Luc harboring Env (white bars) or Env-deficient, bald NL4-3-Luc-R−E− (black bars), washed, and lysed and the p24 content in lysates quantified. The efficiency of p24 binding is shown relative to capture of Env bearing NL4-3 Luc by B-THP DC-SIGN cells, which was set as 100%. The results represent the averages of three independent experiments performed with different virus stocks, and error bars indicate standard errors of the means.
FIG. 5.
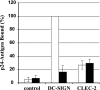
CLEC-2 contributes to HIV-1 capture by platelets. (A) Platelets were incubated with phosphate-buffered saline (PBS), control serum, or anti-CLEC-2 serum and pulsed with HIV-1 NL4-3 Luc generated in 293T cells. After unbound virus was removed, CEMx174 R5 target cells were added and luciferase activities in culture lysates determined 3 days after cocultivation. The averages of seven independent experiments are shown. Error bars indicate standard errors of the means. (B) The transmission experiment was performed as described for panel A, but NL4-3 Luc generated in CEMx174 R5 cells (left panel) or PBMCs (right panel) was employed. The results of representative experiments performed in triplicate are presented, and error bars indicate standard deviations. Similar results were obtained in an independent experiment. (C) HIV-1 capture was assessed as described for panel A, using the indicated primary HIV-1 isolates generated in CEMx174 R5 cells. A representative experiment is shown. The results were confirmed in an independent experiment with a different platelet preparation. Error bars indicate standard deviations. P values were determined using a two-sided (A) independent- or (B and C) dependent-sample t test. IgG, immunoglobulin G; c.p.s., counts per second.
FIG. 6.
A substantial fraction of the HIV-1 capture activity of platelets is dependent on DC-SIGN. (A) The capture assay was carried out as described in the legend for Fig. 5. However, platelets were incubated with rising concentrations of mannan, glucose, or fucose before virus was added. The following carbohydrate concentrations were employed: 0 μg/ml (black bars), 5 μg/ml (dark-gray bars), 10 μg/ml (light-gray bars), and 20 μg/ml (white bars). Similar results were obtained in an independent experiment. (B) HIV-1 capture by platelets was assessed as described for panel A. However, cells were incubated with the indicated inhibitors (MAb 526 blocks ligand binding to DC-SIGN) before addition of virus. Similar results were obtained in three independent experiments. Error bars indicate standard deviations. PBS, phosphate-buffered saline; IgG, immunoglobulin G; c.p.s., counts per second.
FIG. 7.
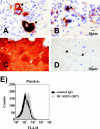
Megakaryocytes (arrows in panels A and B) and platelets (C and D) express DC-SIGN. (A to D) Sections of human bone marrow were immunostained (using the methodology described in the legend for Fig. 2) with anti-DC-SIGN (B and D) or anti-CD61 (A and C). DC-SIGN stains megakaryocytes (B, arrows) and a subpopulation of platelets (D, arrows). (E) Platelets were stained with the indicated anti-DC-SIGN MAb, and staining was analyzed by flow cytometry. Similar results were obtained in three independent experiments with different platelet preparations. IgG, immunoglobulin G; FL4-H, fluorescence in channel 4-height.
FIG. 8.
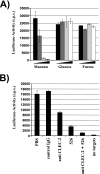
Platelets conserve HIV-1 infectivity in a DC-SIGN- and CLEC-2-dependent manner. HIV-1 NL4-3 Luc was incubated with platelets, cell-free medium (no platelets), or platelets (plat.) preincubated with the indicated inhibitors (MAb 526 blocks ligand binding to DC-SIGN). Subsequently, CEMx174 R5 target cells were added at the indicated time points and luciferase activities in cell lysates determined 3 days after cocultivation. The results are representative of three independent experiments. Error bars indicate standard deviations. IgG, immunoglobulin G; c.p.s., counts per second.
Similar articles
- DC-SIGN-mediated infectious synapse formation enhances X4 HIV-1 transmission from dendritic cells to T cells.
Arrighi JF, Pion M, Garcia E, Escola JM, van Kooyk Y, Geijtenbeek TB, Piguet V. Arrighi JF, et al. J Exp Med. 2004 Nov 15;200(10):1279-88. doi: 10.1084/jem.20041356. J Exp Med. 2004. PMID: 15545354 Free PMC article. - Infection of dendritic cells (DCs), not DC-SIGN-mediated internalization of human immunodeficiency virus, is required for long-term transfer of virus to T cells.
Burleigh L, Lozach PY, Schiffer C, Staropoli I, Pezo V, Porrot F, Canque B, Virelizier JL, Arenzana-Seisdedos F, Amara A. Burleigh L, et al. J Virol. 2006 Mar;80(6):2949-57. doi: 10.1128/JVI.80.6.2949-2957.2006. J Virol. 2006. PMID: 16501104 Free PMC article. - Lentivirus-mediated RNA interference of DC-SIGN expression inhibits human immunodeficiency virus transmission from dendritic cells to T cells.
Arrighi JF, Pion M, Wiznerowicz M, Geijtenbeek TB, Garcia E, Abraham S, Leuba F, Dutoit V, Ducrey-Rundquist O, van Kooyk Y, Trono D, Piguet V. Arrighi JF, et al. J Virol. 2004 Oct;78(20):10848-55. doi: 10.1128/JVI.78.20.10848-10855.2004. J Virol. 2004. PMID: 15452205 Free PMC article. - DC-SIGN points the way to a novel mechanism for HIV-1 transmission.
Masso M. Masso M. MedGenMed. 2003 May 23;5(2):2. MedGenMed. 2003. PMID: 14603101 Review. - DC-SIGN: binding receptors for hepatitis C virus.
Wang QC, Feng ZH, Nie QH, Zhou YX. Wang QC, et al. Chin Med J (Engl). 2004 Sep;117(9):1395-400. Chin Med J (Engl). 2004. PMID: 15377434 Review.
Cited by
- Are Platelets Cells? And if Yes, are They Immune Cells?
Garraud O, Cognasse F. Garraud O, et al. Front Immunol. 2015 Feb 20;6:70. doi: 10.3389/fimmu.2015.00070. eCollection 2015. Front Immunol. 2015. PMID: 25750642 Free PMC article. Review. - The Role of Phlebovirus Glycoproteins in Viral Entry, Assembly and Release.
Spiegel M, Plegge T, Pöhlmann S. Spiegel M, et al. Viruses. 2016 Jul 21;8(7):202. doi: 10.3390/v8070202. Viruses. 2016. PMID: 27455305 Free PMC article. Review. - Haematological Traits in Symptomatic and Asymptomatic COVID-19 Positive Patients for Predicting Severity and Hospitalization.
Alkahtani AM, Alraey Y, Zaman GS, Al-Shehri H, Alghamdi IS, Chandramoorthy HC, Al-Hakami AM, Alamri AM, Alshehri HA. Alkahtani AM, et al. J Blood Med. 2022 Aug 27;13:447-459. doi: 10.2147/JBM.S365218. eCollection 2022. J Blood Med. 2022. PMID: 36062061 Free PMC article. - Beyond basic characterization and omics: Immunomodulatory roles of platelet-derived extracellular vesicles unveiled by functional testing.
Palviainen M, Puutio J, Østergaard RH, Eble JA, Maaninka K, Butt U, Ndika J, Kari OK, Kamali-Moghaddam M, Kjaer-Sorensen K, Oxvig C, Aransay AM, Falcon-Perez JM, Federico A, Greco D, Laitinen S, Hayashi Y, Siljander PR. Palviainen M, et al. J Extracell Vesicles. 2024 Oct;13(10):e12513. doi: 10.1002/jev2.12513. J Extracell Vesicles. 2024. PMID: 39330919 Free PMC article. - The C-type lectin receptors CLEC-2 and Dectin-1, but not DC-SIGN, signal via a novel YXXL-dependent signaling cascade.
Fuller GL, Williams JA, Tomlinson MG, Eble JA, Hanna SL, Pöhlmann S, Suzuki-Inoue K, Ozaki Y, Watson SP, Pearce AC. Fuller GL, et al. J Biol Chem. 2007 Apr 27;282(17):12397-409. doi: 10.1074/jbc.M609558200. Epub 2007 Mar 5. J Biol Chem. 2007. PMID: 17339324 Free PMC article.
References
- Arrighi, J. F., M. Pion, M. Wiznerowicz, T. B. Geijtenbeek, E. Garcia, S. Abraham, F. Leuba, V. Dutoit, O. Ducrey-Rundquist, Y. van Kooyk, D. Trono, and V. Piguet. 2004. Lentivirus-mediated RNA interference of DC-SIGN expression inhibits human immunodeficiency virus transmission from dendritic cells to T cells. J. Virol. 78:10848-10855. - PMC - PubMed
- Baribaud, F., R. W. Doms, and S. Pöhlmann. 2002. The role of DC-SIGN and DC-SIGNR in HIV and Ebola virus infection: can potential therapeutics block virus transmission and dissemination? Expert Opin. Ther. Targets 6:423-431. - PubMed
- Baribaud, F., S. Pöhlmann, T. Sparwasser, M. T. Kimata, Y. K. Choi, B. S. Haggarty, N. Ahmad, T. Macfarlan, T. G. Edwards, G. J. Leslie, J. Arnason, T. A. Reinhart, J. T. Kimata, D. R. Littman, J. A. Hoxie, and R. W. Doms. 2001. Functional and antigenic characterization of human, rhesus macaque, pigtailed macaque, and murine DC-SIGN. J. Virol. 75:10281-10289. - PMC - PubMed
- Bettaieb, A., P. Fromont, F. Louache, E. Oksenhendler, W. Vainchenker, N. Duedari, and P. Bierling. 1992. Presence of cross-reactive antibody between human immunodeficiency virus (HIV) and platelet glycoproteins in HIV-related immune thrombocytopenic purpura. Blood 80:162-169. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases