Evidence that the herpes simplex virus type 1 ICP0 protein does not initiate reactivation from latency in vivo - PubMed (original) (raw)
Evidence that the herpes simplex virus type 1 ICP0 protein does not initiate reactivation from latency in vivo
R L Thompson et al. J Virol. 2006 Nov.
Abstract
The stress-induced host cell factors initiating the expression of the herpes simplex virus lytic cycle from the latent viral genome are not known. Previous studies have focused on the effect of specific viral proteins on reactivation, i.e., the production of detectable infectious virus. However, identification of the viral protein(s) through which host cell factors transduce entry into the lytic cycle and analysis of the promoter(s) of this (these) first protein(s) will provide clues to the identity of the stress-induced host cell factors important for reactivation. In this report, we present the first strategy developed for this type of analysis and use this strategy to test the established hypothesis that the herpes simplex virus ICP0 protein initiates reactivation from the latent state. To this end, ICP0 null and promoter mutants were analyzed for the abilities (i) to exit latency and produce lytic-phase viral proteins (initiate reactivation) and (ii) to produce infectious viral progeny (reactivate) in explant and in vivo. Infection conditions were manipulated so that approximately equal numbers of latent infections were established by the parental strains, the mutants, and their genomically restored counterparts, eliminating disparate latent pool sizes as a complicating factor. Following hyperthermic stress (HS), which induces reactivation in vivo, equivalent numbers of neurons exited latency (as evidenced by the expression of lytic-phase viral proteins) in ganglia latently infected with either the ICP0 null mutant dl1403 or the parental strain. In contrast, infectious virus was detected in the ganglia of mice latently infected with the parental strain but not with ICP0 null mutant dl1403 or FXE. These data demonstrate that the role of ICP0 in the process of reactivation is not as a component of the switch from latency to lytic-phase gene expression; rather, ICP0 is required after entry into the lytic cycle has occurred. Similar analyses were carried out with the DeltaTfi mutant, which contains a 350-bp deletion in the ICP0 promoter, and the genomically restored isolate, DeltaTfiR. The numbers of latently infected neurons exiting latency were not different for DeltaTfi and DeltaTfiR. However, DeltaTfi did not reactivate in vivo, whereas DeltaTfiR reactivated in approximately 38% of the mice. In addition, ICP0 was detected in DeltaTfiR-infected neurons exiting latency but was not detected in those neurons exiting latency infected with DeltaTfi. We conclude that while ICP0 is important and perhaps essential for infectious virus production during reactivation in vivo, this protein is not required and appears to play no major role in the initiation of reactivation in vivo.
Figures
FIG. 1.
Schematic of HSV reactivation from the latent state following stress. Stressful stimuli induce changes within sensory neurons that induce virus reactivation from the latent state. Many studies have shown that infectious virus is produced in latently infected ganglia when explanted in vitro (49). Recently, more-refined analysis at the single-cell level has demonstrated that many thousands of neurons harbor the latent viral genome but that the latent virus in most neurons does not exit the latent state following explant in vitro or hyperthermic stress in vivo. In a very few neurons (<0.1%) viral lytic-phase proteins can be detected, indicating virus entry into the lytic cycle (36, 38, 39, 41-45, 53-55), which we term the initiation of reactivation. Once the latent state has been exited, the lytic-phase proteome is expressed and infectious virus is produced, peaking at 22 h poststress in vivo (44). It has been hypothesized that stress-induced signals operate on the ICP0 promoter and that ICP0 initiates the exit from the latent state by modifying the host cell environment as well as transactivating other viral genes (11, 15, 16).
FIG. 2.
Viral gene transcription during latency and following hyperthermic stress-induced reactivation. (A) RNA related to ICP4, ICP22, ICP47, and ICP27 detected by RT-PCR before and at times indicated after hyperthermic stress. + and − indicate the presence and absence, respectively, of reverse transcriptase in the reaction. RNAs were detected prior to hyperthermic stress within the normally transcribed regions of these genes, as well as upstream of the TATA boxes in the promoters (labeled 5′ of CAP for ICP4; others are not shown). (B) Detection of unspliced ICP0 transcripts and transcripts originating 5′ of the normal ICP0 start site both before and at the indicated times after hyperthermic stress. +RT and No RT indicate the presence and absence, respectively, of reverse transcriptase in the reaction. SKD3 is a mouse ribosomal protein mRNA utilized as a control. Most if not all of the RNA detected is transcribed from somewhere upstream of the ICP0 promoter. (C and D) Total RNA derived from the TG of individual animals (indicated by numbers above the lanes) was assayed for the presence of spliced ICP0 and ICP4 (C) and β-Gal (D) transcripts both before and 1 hour after hyperthermic stress. The results suggest that the ICP0 promoter is upregulated within 1 h poststress.
FIG. 3.
A schematic representation of the viral mutants used in this study. Top: the HSV viral genome with the unique long (UL) and unique short (US) regions as well as the terminal and internal repeat sequences (TR and IR) indicated. The internal repeat region is shown enlarged below, with the mRNAs transcribed within this region indicated by arrows. Short vertical bars indicate the extents of the deletions present in the mutants employed in this study.
FIG. 4.
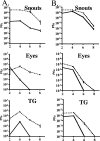
In vivo replication of dl1403 and parental strain 17syn+ (A) and the ΔTfi and ΔTfiR mutants (B). Mice were inoculated on scarified corneas and abraded snouts. At the indicated times, tissues from three mice per inoculation group were examined for infectious virus as described in Materials and Methods. Solid lines represent ICP0 mutants and dashed lines wild type.
FIG. 5.
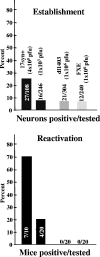
ICP0 null mutants do not reactivate in vivo. Mice were infected on the cornea (strain 17syn+) or the cornea plus the snout (dl1403 and FXE) with the indicated inoculation titers. The percentages of neurons in which latency was established were determined by PCR analysis of individual neurons as described in Materials and Methods. The numbers within the bars are the numbers of neurons positive/tested. Additional animals were subjected to hyperthermic stress, and ganglia were analyzed for infectious virus at 22 h poststress. The percentages of mice positive for infectious virus and the numbers of mice positive/tested are indicated.
FIG. 6.
Schematic representation of the ICP0 gene promoter region and the deletion present in ΔTfi. Top: Base pair numbers relative to the start site of the ICP0 mRNA (horizontal arrow at + 1). Shown are known or potential regulatory elements of the ICP0 gene. Within the 180 bp upstream of the RNA start site is the known Octa/TAATGARAT sequence that confers IE expression kinetics to ICP0 (marked with asterisks), sp1 binding sites, a CAT box, an OLF-1 site, an ICP4 binding site, and a TATA box. Other sites with homology to known factor binding sites, including four additional potential TAATGARAT sites (labeled with question marks), are also indicated. Vertical arrows indicate the limits of the deletion in the ICP0 promoter in the ΔTfi mutant.
FIG. 7.
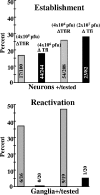
ΔTfi establishes latent infections efficiently but does not reactivate in vivo. Groups of mice were infected with the indicated titers of ΔTfi and ΔTfiR on both scarified corneas and snouts as described in Materials and Methods. The mice were maintained for at least 40 days postinfection. Top: Purified neurons derived from six trigeminal ganglia were analyzed by CXA for the presence of viral genomes as described in Materials and Methods. The gray bars indicate the percentages of neurons that were latently infected with ΔTfiR, and the black bars indicate the percentages latently infected with ΔTfi. The numbers of neurons positive for the viral genome/number of neurons tested are shown for the bars. Bottom: groups of animals were subjected to hyperthermic stress to induce reactivation as described in Materials and Methods. At 22 h postinduction, TG were removed, homogenized, and assayed for the presence of infectious virus. The gray bars indicate the percentages of ΔTfiR-infected animals positive for virus. The black bars indicate the percentages of ΔTfi-infected mice positive for virus. The numbers of animals positive/tested are shown for the bars.
FIG. 8.
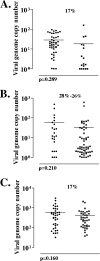
The numbers of viral genome copies present in individual latently infected neurons. The numbers of copies of the viral genome present in each of the positive neurons from Fig. 6 are shown. Each point represents a single neuron. Shown are viral genome copies in neurons from ganglia latently infected with ΔTfi and ΔTfiR when 17% of neurons were latently infected (A), when 28% and 26% of the neurons were latently infected (B), and when 17% of neurons were latently infected but analyzed in groups of 10 neurons instead of individually (C). In this last case, each point represents 10 neurons.
FIG. 9.
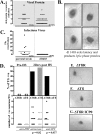
Quantification of the initiation of reactivation from latency at the single-neuron level. Additional mice latently infected with dl1403, ΔTfi, or ΔTfiR were subjected to hyperthermic stress to induce virus reactivation. At 22 h posttreatment, trigeminal ganglia were removed and processed for the immunohistochemical detection of viral proteins as detailed in Materials and Methods. (A) Scattergram showing the numbers of positive neurons detected in individual ganglia from animals latently infected with dl1403 or with the parental strain. Seventeen of 37 ganglia tested from dl1403-infected mice contained one or more positive (+ve) neurons. (B) Photomicrographs of four representative neurons expressing viral proteins in the ganglia of mice latently infected with dl1403 at 22 h after hyperthermic stress (brown precipitate). (C) None of 56 ganglia tested from dl1403-infected mice were positive for virus. (D) Percentages of ganglia containing viral protein-positive neurons from mice latently infected with ΔTfi and ΔTfiR before and 22 h after hyperthermic stress (top), and numbers of positive neurons in each ganglion (bottom). The numbers of viral protein-positive neurons detected in the ganglia of mice infected with ΔTfi and with ΔTfiR were similar poststress (P = 0.82). However, ICP0 was not detected in ganglia from ΔTfi group (P = 0.037). (E, F, and G) Photomicrographs of representative neurons in latently infected ganglia immunohistochemically stained for lytic viral proteins (E and F) or for ICP0 (G) at 22 h after hyperthermic stress are shown.
Similar articles
- Mutations Inactivating Herpes Simplex Virus 1 MicroRNA miR-H2 Do Not Detectably Increase ICP0 Gene Expression in Infected Cultured Cells or Mouse Trigeminal Ganglia.
Pan D, Pesola JM, Li G, McCarron S, Coen DM. Pan D, et al. J Virol. 2017 Jan 3;91(2):e02001-16. doi: 10.1128/JVI.02001-16. Print 2017 Jan 15. J Virol. 2017. PMID: 27847363 Free PMC article. - ICP0 is required for efficient reactivation of herpes simplex virus type 1 from neuronal latency.
Halford WP, Schaffer PA. Halford WP, et al. J Virol. 2001 Apr;75(7):3240-9. doi: 10.1128/JVI.75.7.3240-3249.2001. J Virol. 2001. PMID: 11238850 Free PMC article. - Optimized viral dose and transient immunosuppression enable herpes simplex virus ICP0-null mutants To establish wild-type levels of latency in vivo.
Halford WP, Schaffer PA. Halford WP, et al. J Virol. 2000 Jul;74(13):5957-67. doi: 10.1128/jvi.74.13.5957-5967.2000. J Virol. 2000. PMID: 10846077 Free PMC article. - ICP0, a regulator of herpes simplex virus during lytic and latent infection.
Everett RD. Everett RD. Bioessays. 2000 Aug;22(8):761-70. doi: 10.1002/1521-1878(200008)22:8<761::AID-BIES10>3.0.CO;2-A. Bioessays. 2000. PMID: 10918307 Review. - A comparison of herpes simplex virus type 1 and varicella-zoster virus latency and reactivation.
Kennedy PG, Rovnak J, Badani H, Cohrs RJ. Kennedy PG, et al. J Gen Virol. 2015 Jul;96(Pt 7):1581-602. doi: 10.1099/vir.0.000128. Epub 2015 Mar 20. J Gen Virol. 2015. PMID: 25794504 Free PMC article. Review.
Cited by
- Herpes simplex virus and the lexicon of latency and reactivation: a call for defining terms and building an integrated collective framework.
Sawtell NM, Thompson RL. Sawtell NM, et al. F1000Res. 2016 Aug 19;5:F1000 Faculty Rev-2038. doi: 10.12688/f1000research.8886.1. eCollection 2016. F1000Res. 2016. PMID: 27610228 Free PMC article. Review. - Viral Ubiquitin Ligase Stimulates Selective Host MicroRNA Expression by Targeting ZEB Transcriptional Repressors.
Lutz G, Jurak I, Kim ET, Kim JY, Hackenberg M, Leader A, Stoller ML, Fekete DM, Weitzman MD, Coen DM, Wilson AC. Lutz G, et al. Viruses. 2017 Aug 7;9(8):210. doi: 10.3390/v9080210. Viruses. 2017. PMID: 28783105 Free PMC article. - Interactome and Ubiquitinome Analyses Identify Functional Targets of Herpes Simplex Virus 1 Infected Cell Protein 0.
Hou F, Sun Z, Deng Y, Chen S, Yang X, Ji F, Zhou M, Ren K, Pan D. Hou F, et al. Front Microbiol. 2022 Apr 18;13:856471. doi: 10.3389/fmicb.2022.856471. eCollection 2022. Front Microbiol. 2022. PMID: 35516420 Free PMC article. - A viral E3 ligase targets RNF8 and RNF168 to control histone ubiquitination and DNA damage responses.
Lilley CE, Chaurushiya MS, Boutell C, Landry S, Suh J, Panier S, Everett RD, Stewart GS, Durocher D, Weitzman MD. Lilley CE, et al. EMBO J. 2010 Mar 3;29(5):943-55. doi: 10.1038/emboj.2009.400. Epub 2010 Jan 14. EMBO J. 2010. PMID: 20075863 Free PMC article. - The zinc RING finger of bovine herpesvirus 1-encoded bICP0 protein is crucial for viral replication and virulence.
Saira K, Chowdhury S, Gaudreault N, da Silva L, Henderson G, Doster A, Jones C. Saira K, et al. J Virol. 2008 Dec;82(24):12060-8. doi: 10.1128/JVI.01348-08. Epub 2008 Oct 8. J Virol. 2008. PMID: 18842710 Free PMC article.
References
- Chen, S. H., L. Y. Lee, D. A. Garber, P. A. Schaffer, D. M. Knipe, and D. M. Coen. 2002. Neither LAT nor open reading frame P mutations increase expression of spliced or intron-containing ICP0 transcripts in mouse ganglia latently infected with herpes simplex virus. J. Virol. 76:4764-4772. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI032121/AI/NIAID NIH HHS/United States
- R01 EY013168/EY/NEI NIH HHS/United States
- 5R01 AI 32121/AI/NIAID NIH HHS/United States
- 5R01 EY 13168/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources