Nup214 is required for CRM1-dependent nuclear protein export in vivo - PubMed (original) (raw)
Nup214 is required for CRM1-dependent nuclear protein export in vivo
Saskia Hutten et al. Mol Cell Biol. 2006 Sep.
Abstract
Nucleoporins mediate transport of macromolecules across the nuclear pore complex, yet the function of many individual nucleoporins is largely unresolved. To address this question, we depleted cells of the cytoplasmic nucleoporins Nup214/CAN and Nup358/RanBP2 by RNA interference. Depletion of Nup214 resulted in codepletion of its binding partner, Nup88. Nuclear pore complexes assembled in the absence of Nup214/Nup88 or Nup358 were fully functional in nuclear protein import, whereas nuclear mRNA export was slightly impaired. Depletion of Nup358 had only a minor effect on nuclear protein export. In contrast, depletion of Nup214/Nup88 led to strongly reduced CRM1-mediated export of the shuttling transcription factor NFAT as well as a human immunodeficiency virus-Rev derivative. A specific role of Nup214 in protein export is furthered by the biochemical properties of a high-affinity complex containing Nup214, CRM1, RanGTP, and an export cargo. Our results show that the Nup214/Nup88 complex is required for efficient CRM1-mediated transport, supporting a model involving a high-affinity binding site for CRM1 at Nup214 in the terminal steps of export.
Figures
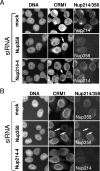
FIG. 1.
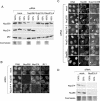
Efficient and specific depletion of Nup214 and Nup358 by RNA interference. HeLa cells were mock treated or transfected with specific siRNAs against Nup214 or Nup358. (A) Different amounts (100%, 50%, 25%) of cell lysates from mock-treated cells were compared to lysates from nucleoporin-depleted cells by immunoblotting, detecting Nup358, Nup214, and p62. A parallel Coomassie stain served as a loading control. Note the slightly lower protein concentration in the lysate obtained from Nup214-2-transfected cells. (B) Mock- or siRNA-treated cells were analyzed by triple immunofluorescence, using anti-Nup358, anti-Nup214, and the antinucleoporin antibody RL1, as indicated. (C) Mock- or siRNA-treated cells were analyzed by double immunofluorescence for Nup88 and Nup214 or Nup358. Nucleoporin-depleted cells are indicated by arrows. For a better comparison of protein levels, nucleoporin-depleted cells were mixed with untransfected cells. Antibodies used for detection of Nup214 or Nup358 are indicated in the individual pictures (right panel). (D) Different amounts (100%, 50%) of cell lysates from mock-treated cells and Nup214-depleted cells were analyzed by immunoblotting, detecting Nup214 and Nup88. A parallel Coomassie stain served as a loading control. Note that our commercial anti-Nup88 antibody did not allow a reliable quantitative analysis of the codepletion.
FIG. 2.
Reduced cell growth in Nup358- and Nup214-depleted cells. (A) Triplicates of mock-treated or nucleoporin-depleted cells were trypsinized and counted at various time points (days after transfection) during the siRNA treatment. Error bars indicate the standard deviation from the mean. (B) Mock- or siRNA-treated cells were analyzed by propidium iodide stain for cell cycle distribution and analyzed by flow cytometry. Percentages of cells in individual stages of the cell cycle are indicated.
FIG. 3.
Poly(A)+ RNA export is slightly impaired in Nup358- or Nup214-depleted cells. Mock-treated or Nup214- or Nup358-depleted cells were analyzed by in situ hybridization for poly(A)+ RNA and in parallel by indirect immunofluorescence for the nucleoporins, as indicated. For a better comparison of nucleoporin levels, depleted cells were mixed with untransfected cells. Nucleoporin-depleted cells showed either the same pattern as mock-treated cells (arrows) or an equal distribution of poly(A)+ RNA between the nucleus and the cytoplasm (arrowheads). No signal was obtained with the control oligonucleotide oligo(dA) (lower panel).
FIG. 4.
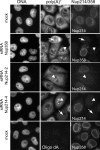
Nup214 and Nup358 are not required for nuclear protein import. Nup214- or Nup358-depleted HeLa cells were transiently transfected with constructs coding for GFP-GFP-NLS (A) or GFP-GFP-M9 (B), stained for Nup214 and Nup358, and analyzed by fluorescence microscopy. (C) Mock-treated or Nup214- or Nup358-depleted HeLa-GFP-NFAT cells were fixed before (−ionomycin) or after (+ionomycin) treatment with ionomycin, stained for residual nucleoporins at the NPC, and analyzed by fluorescence microscopy. For a better comparison of RNAi and transport efficiencies, nucleoporin-depleted HeLa-GFP-NFAT cells were mixed with untransfected cells. (A to C) Antibodies used for detection of Nup214 or Nup358 are indicated in the individual pictures (right panels).
FIG. 5.
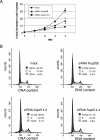
Depletion of Nup214 inhibits nuclear export of GFP-NFAT. HeLa-GFP-NFAT cells were either mock treated or transfected with siRNAs against Nup214 or Nup358 and subjected to one import/export cycle. Cells were stained for Nup358 or Nup214 and analyzed by fluorescence microscopy for GFP-NFAT distribution. For a better comparison of RNAi and transport efficiencies, nucleoporin-depleted cells were mixed with untransfected cells. (A) Nup214-depleted cells showed either a nuclear accumulation (arrows) or an equal distribution of GFP-NFAT between nucleus and cytoplasm (arrowhead). Export reactions were stopped after 90 min (mock; siRNA Nup358 and siRNA Nup214-4) or 120 min (siRNA Nup214-2). The mean distribution of cells in the three categories N > C, N = C, and N < C for GFP-NFAT fluorescence is shown for Nup358-depleted cells (B) and for Nup214-depleted cells (C). Bars indicate the standard deviation from the mean of three independent experiments. (D) Time course of cytoplasmic accumulation (N < C) of GFP-NFAT in mock-treated, untransfected, and Nup214-depleted cells (siRNA Nup214-2). Bars indicate the variation of the individual values from the mean of two independent experiments. (B to D) Only efficiently depleted cells (≥100 per experiment) were included in the analyses. Cells transfected with siRNA Nup214-2 (A and D) were analyzed at day 2 without retransfection. (E) Detection of GFP-NFAT in total cell lysates of mock-treated or Nup214-depleted cells before import (−ion), after import (+ion), and after export (exp) reactions by immunoblotting, using an anti-GFP antibody.
FIG. 6.
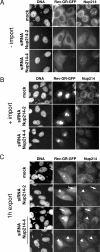
Depletion of Nup214 inhibits nuclear export of Rev-GR-GFP. Mock-treated or Nup214-depleted HeLa cells were transfected with a vector coding for Rev-GR-GFP and subjected to transport reactions. Cells were fixed before (A, −import), after the addition of dexamethasone (B, +import), or after the export reaction (C, 1 h export) and analyzed by fluorescence microscopy. Nup214-depleted cells showed either an equal distribution of Rev-GR-GFP between nucleus and cytoplasm (arrow) or a nuclear accumulation of the reporter (arrowhead).
FIG. 7.
Cytoplasmic nucleoporins are not required for efficient CRM1 import. Mock- or siRNA-treated cells were permeabilized and incubated with recombinant His-CRM1-Cy2 for (A) 30 min at 30°C or (B) in the presence of WGA for 30 min at 4°C. Cells were stained for Nup358 or Nup214 and analyzed by fluorescence microscopy. (A) Arrows point to nucleoporin-depleted cells, showing CRM1 import similar to that of control cells. (B) Arrows point to Nup358-depleted cells with reduced nuclear rim for CRM1. For a better comparison of protein levels, nucleoporin-depleted cells were mixed with untransfected cells.
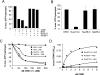
FIG. 8.
CRM1 forms a high-affinity, RanGAP-resistant complex with RanGTP, Nup214, and export cargo. (A) RanGAP assays were performed with 220 nM CRM1 in the absence or presence of 40 μM NES peptide, 800 nM Nup214 fragment, and 1.1 μM RanBP1, as indicated. (B) RanGAP assays were performed with 240 nM CRM1 in the absence or presence of 450 nM Nup214-C, 2 μM Nup88-N, or 2.2 μM Nup88-C, as indicated. Error bars correspond to the standard deviations from the means of three (Nup214) or five (Nup88) independent experiments. (C) RanGAP assays were performed with NES peptide or Nup214 fragment as described for panel A and increasing concentrations of CRM1 that had been preincubated with (+LMB) or without (−LMB) leptomycin B, as indicated. (D) Solid-phase binding of CRM1 to Nup214 was analyzed in the absence or presence of RanGTPγS, RanGDP, or NES peptide, as indicated. OD450, optical density at 450 nm; max., maximum.
Similar articles
- The nucleoporin Nup214 sequesters CRM1 at the nuclear rim and modulates NFkappaB activation in Drosophila.
Xylourgidis N, Roth P, Sabri N, Tsarouhas V, Samakovlis C. Xylourgidis N, et al. J Cell Sci. 2006 Nov 1;119(Pt 21):4409-19. doi: 10.1242/jcs.03201. Epub 2006 Oct 10. J Cell Sci. 2006. PMID: 17032737 - The nuclear pore component Nup358 promotes transportin-dependent nuclear import.
Hutten S, Wälde S, Spillner C, Hauber J, Kehlenbach RH. Hutten S, et al. J Cell Sci. 2009 Apr 15;122(Pt 8):1100-10. doi: 10.1242/jcs.040154. Epub 2009 Mar 19. J Cell Sci. 2009. PMID: 19299463 - Supraphysiological nuclear export signals bind CRM1 independently of RanGTP and arrest at Nup358.
Engelsma D, Bernad R, Calafat J, Fornerod M. Engelsma D, et al. EMBO J. 2004 Sep 15;23(18):3643-52. doi: 10.1038/sj.emboj.7600370. Epub 2004 Aug 26. EMBO J. 2004. PMID: 15329671 Free PMC article. - Leucine-rich nuclear-export signals: born to be weak.
Kutay U, Güttinger S. Kutay U, et al. Trends Cell Biol. 2005 Mar;15(3):121-4. doi: 10.1016/j.tcb.2005.01.005. Trends Cell Biol. 2005. PMID: 15752974 Review. - Mechanisms of receptor-mediated nuclear import and nuclear export.
Pemberton LF, Paschal BM. Pemberton LF, et al. Traffic. 2005 Mar;6(3):187-98. doi: 10.1111/j.1600-0854.2005.00270.x. Traffic. 2005. PMID: 15702987 Review.
Cited by
- RNA Export through the NPC in Eukaryotes.
Okamura M, Inose H, Masuda S. Okamura M, et al. Genes (Basel). 2015 Mar 20;6(1):124-49. doi: 10.3390/genes6010124. Genes (Basel). 2015. PMID: 25802992 Free PMC article. Review. - Tough Way In, Tough Way Out: The Complex Interplay of Host and Viral Factors in Nucleocytoplasmic Trafficking during HIV-1 Infection.
Sarkar S, Balakrishnan K, Chintala K, Mohareer K, Luedde T, Vasudevan AAJ, Münk C, Banerjee S. Sarkar S, et al. Viruses. 2022 Nov 12;14(11):2503. doi: 10.3390/v14112503. Viruses. 2022. PMID: 36423112 Free PMC article. Review. - Inhibition of CRM1-mediated nuclear export of transcription factors by leukemogenic NUP98 fusion proteins.
Takeda A, Sarma NJ, Abdul-Nabi AM, Yaseen NR. Takeda A, et al. J Biol Chem. 2010 May 21;285(21):16248-57. doi: 10.1074/jbc.M109.048785. Epub 2010 Mar 16. J Biol Chem. 2010. PMID: 20233715 Free PMC article. - Nuclear export factor RBM15 facilitates the access of DBP5 to mRNA.
Zolotukhin AS, Uranishi H, Lindtner S, Bear J, Pavlakis GN, Felber BK. Zolotukhin AS, et al. Nucleic Acids Res. 2009 Nov;37(21):7151-62. doi: 10.1093/nar/gkp782. Nucleic Acids Res. 2009. PMID: 19786495 Free PMC article. - The nuclear pore proteins Nup88/214 and T-cell acute lymphatic leukemia-associated NUP214 fusion proteins regulate Notch signaling.
Kindermann B, Valkova C, Krämer A, Perner B, Engelmann C, Behrendt L, Kritsch D, Jungnickel B, Kehlenbach RH, Oswald F, Englert C, Kaether C. Kindermann B, et al. J Biol Chem. 2019 Aug 2;294(31):11741-11750. doi: 10.1074/jbc.RA118.006357. Epub 2019 Jun 11. J Biol Chem. 2019. PMID: 31186352 Free PMC article.
References
- Bachi, A., I. C. Braun, J. P. Rodrigues, N. Pante, K. Ribbeck, C. von Kobbe, U. Kutay, M. Wilm, D. Görlich, M. Carmo-Fonseca, and E. Izaurralde. 2000. The C-terminal domain of TAP interacts with the nuclear pore complex and promotes export of specific CTE-bearing RNA substrates. RNA 6:136-158. - PMC - PubMed
- Beals, C. R., N. A. Clipstone, S. N. Ho, and G. R. Crabtree. 1997. Nuclear localization of NF-ATc by a calcineurin-dependent, cyclosporin-sensitive intramolecular interaction. Genes Dev. 11:824-834. - PubMed
- Beals, C. R., C. M. Sheridan, C. W. Turck, P. Gardner, and G. R. Crabtree. 1997. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science 275:1930-1934. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous