Consistent resting-state networks across healthy subjects - PubMed (original) (raw)
Consistent resting-state networks across healthy subjects
J S Damoiseaux et al. Proc Natl Acad Sci U S A. 2006.
Abstract
Functional MRI (fMRI) can be applied to study the functional connectivity of the human brain. It has been suggested that fluctuations in the blood oxygenation level-dependent (BOLD) signal during rest reflect the neuronal baseline activity of the brain, representing the state of the human brain in the absence of goal-directed neuronal action and external input, and that these slow fluctuations correspond to functionally relevant resting-state networks. Several studies on resting fMRI have been conducted, reporting an apparent similarity between the identified patterns. The spatial consistency of these resting patterns, however, has not yet been evaluated and quantified. In this study, we apply a data analysis approach called tensor probabilistic independent component analysis to resting-state fMRI data to find coherencies that are consistent across subjects and sessions. We characterize and quantify the consistency of these effects by using a bootstrapping approach, and we estimate the BOLD amplitude modulation as well as the voxel-wise cross-subject variation. The analysis found 10 patterns with potential functional relevance, consisting of regions known to be involved in motor function, visual processing, executive functioning, auditory processing, memory, and the so-called default-mode network, each with BOLD signal changes up to 3%. In general, areas with a high mean percentage BOLD signal are consistent and show the least variation around the mean. These findings show that the baseline activity of the brain is consistent across subjects exhibiting significant temporal dynamics, with percentage BOLD signal change comparable with the signal changes found in task-related experiments.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
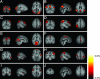
Fig. 1.
Tensor-PICA estimated resting patterns of the first (A–J) and second (A′–I′ and K) multisubject data sets: coronal, sagittal, and axial view of spatial map for each component. A–I and A′–I′ show components found in both data sets. J and K components are unique to their data sets. Images are z statistics overlaid on the average high-resolution scan transformed into standard (MNI152) space. Black to yellow are z values, ranging from 2.0 to 5.0. The left hemisphere of the brain corresponds to the right side of the image.
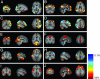
Fig. 2.
Mean (across 100 surrogate multisubject data sets) tensor-PICA estimated resting patterns: coronal, sagittal, and axial view of spatial map for each component. Images are percentage BOLD signal change, overlaid on the average high-resolution scan transformed into standard (MNI152) space. Black to yellow is percentage signal change, ranging from 0.5% to 3.0%.
Fig. 3.
Maps of coefficient of variation: coronal, sagittal, and axial view of spatial map for each component. Images are percentage variation around the mean percentage BOLD signal change, overlaid on the average high-resolution scan transformed into standard (MNI152) space. Red denotes much variation around the mean; blue denotes little variation.
Similar articles
- Spatiotemporal dynamics of the brain at rest--exploring EEG microstates as electrophysiological signatures of BOLD resting state networks.
Yuan H, Zotev V, Phillips R, Drevets WC, Bodurka J. Yuan H, et al. Neuroimage. 2012 May 1;60(4):2062-72. doi: 10.1016/j.neuroimage.2012.02.031. Epub 2012 Feb 22. Neuroimage. 2012. PMID: 22381593 - [Functional connectivity analysis of the brain network using resting-state FMRI].
Hayashi T. Hayashi T. Brain Nerve. 2011 Dec;63(12):1307-18. Brain Nerve. 2011. PMID: 22147450 Review. Japanese. - Detecting resting-state brain activity by spontaneous cerebral blood volume fluctuations using whole brain vascular space occupancy imaging.
Miao X, Gu H, Yan L, Lu H, Wang DJ, Zhou XJ, Zhuo Y, Yang Y. Miao X, et al. Neuroimage. 2014 Jan 1;84:575-84. doi: 10.1016/j.neuroimage.2013.09.019. Epub 2013 Sep 20. Neuroimage. 2014. PMID: 24055705 - Principal components of functional connectivity: a new approach to study dynamic brain connectivity during rest.
Leonardi N, Richiardi J, Gschwind M, Simioni S, Annoni JM, Schluep M, Vuilleumier P, Van De Ville D. Leonardi N, et al. Neuroimage. 2013 Dec;83:937-50. doi: 10.1016/j.neuroimage.2013.07.019. Epub 2013 Jul 18. Neuroimage. 2013. PMID: 23872496 - Using resting state functional connectivity to unravel networks of tinnitus.
Husain FT, Schmidt SA. Husain FT, et al. Hear Res. 2014 Jan;307:153-62. doi: 10.1016/j.heares.2013.07.010. Epub 2013 Jul 26. Hear Res. 2014. PMID: 23895873 Review.
Cited by
- Shedding Light on the Aftermath: Childhood Maltreatment's Role in Modifying the Association Between Recent Life Stress and Resting-State Network Connectivity.
Luo J, Zhu J, The Nspn Consortium, Chen Y. Luo J, et al. Behav Sci (Basel). 2024 Oct 16;14(10):958. doi: 10.3390/bs14100958. Behav Sci (Basel). 2024. PMID: 39457830 Free PMC article. - Microstates in resting-state EEG: current status and future directions.
Khanna A, Pascual-Leone A, Michel CM, Farzan F. Khanna A, et al. Neurosci Biobehav Rev. 2015 Feb;49:105-13. doi: 10.1016/j.neubiorev.2014.12.010. Epub 2014 Dec 17. Neurosci Biobehav Rev. 2015. PMID: 25526823 Free PMC article. Review. - Gender differences in cerebral regional homogeneity of adult healthy volunteers: a resting-state FMRI study.
Xu C, Li C, Wu H, Wu Y, Hu S, Zhu Y, Zhang W, Wang L, Zhu S, Liu J, Zhang Q, Yang J, Zhang X. Xu C, et al. Biomed Res Int. 2015;2015:183074. doi: 10.1155/2015/183074. Epub 2015 Jan 1. Biomed Res Int. 2015. PMID: 25629038 Free PMC article. - Effects of scanner acoustic noise on intrinsic brain activity during auditory stimulation.
Yakunina N, Kang EK, Kim TS, Min JH, Kim SS, Nam EC. Yakunina N, et al. Neuroradiology. 2015 Oct;57(10):1063-73. doi: 10.1007/s00234-015-1561-1. Epub 2015 Jul 21. Neuroradiology. 2015. PMID: 26193957 - Insular Dysfunction Reflects Altered Between-Network Connectivity and Severity of Negative Symptoms in Schizophrenia during Psychotic Remission.
Manoliu A, Riedl V, Doll A, Bäuml JG, Mühlau M, Schwerthöffer D, Scherr M, Zimmer C, Förstl H, Bäuml J, Wohlschläger AM, Koch K, Sorg C. Manoliu A, et al. Front Hum Neurosci. 2013 May 20;7:216. doi: 10.3389/fnhum.2013.00216. eCollection 2013. Front Hum Neurosci. 2013. PMID: 23730284 Free PMC article.
References
- Lowe MJ, Dzemidzic M, Lurito JT, Mathews VP, Phillips MD. NeuroImage. 2000;12:582–587. - PubMed
- Obrig H, Neufang M, Wenzel R, Kohl M, Steinbrink J, Einhaupl K, Villringer A. NeuroImage. 2000;12:623–639. - PubMed
- Wise RG, Ide K, Poulin MJ, Tracey I. NeuroImage. 2004;21:1652–1664. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources