Differential expression of neuronal ACE2 in transgenic mice with overexpression of the brain renin-angiotensin system - PubMed (original) (raw)
Differential expression of neuronal ACE2 in transgenic mice with overexpression of the brain renin-angiotensin system
Marc F Doobay et al. Am J Physiol Regul Integr Comp Physiol. 2007 Jan.
Abstract
Angiotensin-converting enzyme 2 (ACE2) is a newly discovered carboxy-peptidase responsible for the formation of vasodilatory peptides such as angiotensin-(1-7). We hypothesized that ACE2 is part of the brain renin-angiotensin system, and its expression is regulated by the other elements of this system. ACE2 immunostaining was performed in transgenic mouse brain sections from neuron-specific enolase-AT(1A) (overexpressing AT(1A) receptors), R(+)A(+) (overexpressing angiotensinogen and renin), and control (nontransgenic littermates) mice. Results show that ACE2 staining is widely distributed throughout the brain. Using cell-type-specific antibodies, we observed that ACE2 staining is present in the cytoplasm of neuronal cell bodies but not in glial cells. In the subfornical organ, an area lacking the blood-brain barrier and sensitive to blood-borne angiotensin II, ACE2 was significantly increased in transgenic mice. Interestingly, ACE2 mRNA and protein expression were inversely correlated in the nucleus of tractus solitarius/dorsal motor nucleus of the vagus and the ventrolateral medulla, when comparing transgenic to nontransgenic mice. These results suggest that ACE2 is localized to the cytoplasm of neuronal cells in the brain and that ACE2 levels appear highly regulated by other components of the renin-angiotensin system, confirming its involvement in this system. Moreover, ACE2 expression in brain structures involved in the control of cardiovascular function suggests that the carboxypeptidase may have a role in the central regulation of blood pressure and diseases involving the autonomic nervous system, such as hypertension.
Figures
Figure 1
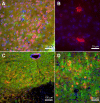
ACE2 is expressed in the cytoplasm of neuronal cells. (A) Immuno-staining for ACE2 (red), GFAP (glial cell marker, green), and DAPI (nucleic acid marker, blue) in the piriform cortex shows a lack of co-localization between ACE2 and the astroglia (magnification 40X). (B) Increased magnification (63X) in the caudate putamen shows the absence of GFAP staining in ACE2-positive cells. Immuno-reactivity is localized in the cytoplasm and not in the nucleus. Immuno-staining for ACE2, MAP2 (neuronal marker, green), and DAPI in the hypoglossal nucleus (C) and the primary motor cortex (D) shows co-localization (yellow) between ACE2 and MAP2 suggesting that ACE2 is expressed mostly in neurons. All panels are from C57BL/6J mice.
Figure 2
ACE2 quantification in mouse brain. Levels of ACE2 fluorescence were graded throughout the whole brain using the following: no detectable immunostaining (-), low-level immunostaining (+), abundant immunostaining (++), and highly abundant immunostaining (+++). All panels are from C57BL/6J mice.
Figure 3
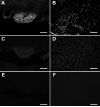
Specificity of the ACE2 antibody. Brain sections of the subfornical organ (SFO; A,C,E) and rostral ventrolateral medulla (RVLM; B,D,F) from C57BL/6J (A,B) and ACE2-/y (C-F) mice were incubated with (A-D) and without (E,F) anti-ACE2 antibody. Note that ACE2 immunostaining is restricted to the SFO (A) and absent in the surrounding tissue. The lack of ACE2 immunostaining in ACE2-/y mice (C-F) confirms the specificity of the anti-ACE2 antibody (scale bar: 100 μm).
Figure 4
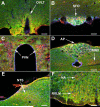
ACE2 expression in key brain regions involved in the regulation of blood pressure and body fluid homeostasis. This panel shows examples of ACE2 (red) expression in (A) the organum vasculosum of the lamina terminalis (OVLT), an area involved in thirst and salt-appetite. Brain nuclei involved in the regulation of cardiovascular function such as (B) the subfornical organ (SFO), (C) the magnocellular neurons of the paraventricular nucleus (PVN), (D) the area postrema (AP), the dorsal motor nucleus of the vagus (DMNX), (E) the nucleus of tractus solitarii (NTS), (F) the rostroventrolateral medulla (RVLM), and the nucleus ambiguus (NA) also showed positive staining for ACE2. The neuronal marker MAP2 is shown in green and cell nuclei are stained in blue. All panels are from C57BL/6J mice (scale bar: 100 μm).
Figure 5
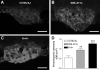
ACE2 protein expression in the forebrain is genotype-dependent. Typical examples in the subfornical organ (SFO) of the differential expression of ACE2 in C57BL6/J (A), NSE-AT1A (B) and R+A+ mice (C). Quantification of ACE2 immunoreactivity (D) shows that expression of the enzyme is modulated by overexpression of central AT1A receptors in NSE-AT1A mice and/or human renin and human AGT in R+A+ mice in the SFO. **P<0.01 and ***P<0.001 versus non-transgenic mice (scale bar: 100 μm).
Figure 6
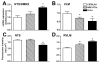
ACE2 is differentially expressed in transgenic mouse brainstem. Quantification of ACE2 mRNA expression in the nucleus of tractus solitarius/dorsal motor nucleus of the vagus (NTS/DMNX) (A) and the ventrolateral medulla (VLM) (B) and protein immunostaining in the NTS (C) and the rostral ventrolateral medulla (RVLM) (D). Immunostaining and mRNA expression are inversely correlated in these areas and regulated by the other components of the RAS. *P<0.05 versus C57BL6/J mice.
Similar articles
- Brain-selective overexpression of human Angiotensin-converting enzyme type 2 attenuates neurogenic hypertension.
Feng Y, Xia H, Cai Y, Halabi CM, Becker LK, Santos RA, Speth RC, Sigmund CD, Lazartigues E. Feng Y, et al. Circ Res. 2010 Feb 5;106(2):373-82. doi: 10.1161/CIRCRESAHA.109.208645. Epub 2009 Nov 19. Circ Res. 2010. PMID: 19926873 Free PMC article. - Angiotensin II type 1 receptor-mediated reduction of angiotensin-converting enzyme 2 activity in the brain impairs baroreflex function in hypertensive mice.
Xia H, Feng Y, Obr TD, Hickman PJ, Lazartigues E. Xia H, et al. Hypertension. 2009 Feb;53(2):210-6. doi: 10.1161/HYPERTENSIONAHA.108.123844. Epub 2009 Jan 5. Hypertension. 2009. PMID: 19124678 Free PMC article. - Brain angiotensin-converting enzyme type 2 shedding contributes to the development of neurogenic hypertension.
Xia H, Sriramula S, Chhabra KH, Lazartigues E. Xia H, et al. Circ Res. 2013 Oct 12;113(9):1087-1096. doi: 10.1161/CIRCRESAHA.113.301811. Epub 2013 Sep 6. Circ Res. 2013. PMID: 24014829 Free PMC article. - The two fACEs of the tissue renin-angiotensin systems: implication in cardiovascular diseases.
Lazartigues E, Feng Y, Lavoie JL. Lazartigues E, et al. Curr Pharm Des. 2007;13(12):1231-45. doi: 10.2174/138161207780618911. Curr Pharm Des. 2007. PMID: 17504232 Review. - [The importance of ACE2 in regulating the cardiovascular system].
Raz A, Gamliel-Lazarovich A, Bogner I, Strigevsky A, Keidar S. Raz A, et al. Harefuah. 2007 Sep;146(9):703-6, 733. Harefuah. 2007. PMID: 17969309 Review. Hebrew.
Cited by
- The COVID-19 pandemic and Alzheimer's disease: mutual risks and mechanisms.
Chen F, Chen Y, Wang Y, Ke Q, Cui L. Chen F, et al. Transl Neurodegener. 2022 Sep 11;11(1):40. doi: 10.1186/s40035-022-00316-y. Transl Neurodegener. 2022. PMID: 36089575 Free PMC article. Review. - Targeting of renin-angiotensin system in COVID-19 patients affected by stroke: Emerging concerns about detrimental vs. benefit effect.
Luzzi S, Giotta Lucifero A, Marasco S, Del Maestro M, Bellantoni G, Gragnaniello C. Luzzi S, et al. Interdiscip Neurosurg. 2020 Dec;22:100822. doi: 10.1016/j.inat.2020.100822. Epub 2020 Jul 10. Interdiscip Neurosurg. 2020. PMID: 32835018 Free PMC article. - Acute and chronic neuropsychiatric symptoms in novel coronavirus disease 2019 (COVID-19) patients: A qualitative review.
Smith CJ, Renshaw P, Yurgelun-Todd D, Sheth C. Smith CJ, et al. Front Public Health. 2022 Aug 8;10:772335. doi: 10.3389/fpubh.2022.772335. eCollection 2022. Front Public Health. 2022. PMID: 36033820 Free PMC article. Review. - Heteromeric Solute Carriers: Function, Structure, Pathology and Pharmacology.
Fairweather SJ, Shah N, Brӧer S. Fairweather SJ, et al. Adv Exp Med Biol. 2021;21:13-127. doi: 10.1007/5584_2020_584. Adv Exp Med Biol. 2021. PMID: 33052588 Review. - A Review on Headaches Due to COVID-19 Infection.
Togha M, Hashemi SM, Yamani N, Martami F, Salami Z. Togha M, et al. Front Neurol. 2022 Jul 12;13:942956. doi: 10.3389/fneur.2022.942956. eCollection 2022. Front Neurol. 2022. PMID: 35911910 Free PMC article. Review.
References
- Allen AM, Zhuo J, Mendelsohn FAO. Localization and function of angiotensin AT1 receptors. Am J Hypertens. 2000;13:31S–38S. - PubMed
- Averill DB, Diz DI. Angiotensin peptides and baroreflex control of sympathetic outflow: pathways and mechanisms of the medulla oblongata. Brain Res Bull. 2000;51:119–128. - PubMed
- Bader M, Peters J, Baltatu O, Muller DN, Luft FC, Ganten D. Tissue renin-angiotensin systems: new insights from experimental animal models in hypertension research. J Mol Med. 2001;79:76–102. - PubMed
- Banegas I, Prieto I, Alba F, Vives F, Araque A, Segarra AB, Duran R, De Gasparo M, Ramirez M. Angiotensinase activity is asymmetrically distributed in the amygdala, hippocampus and prefrontal cortex of the rat. Behav Brain Res. 2005;156:321–326. - PubMed
- Benter IF, Diz DI, Ferrario CM. Pressor and reflex sensitivity is altered in spontaneously hypertensive rats treated with angiotensin-(1-7) Hypertension. 1995;26:1138–1144. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases