Cholangiocyte cilia detect changes in luminal fluid flow and transmit them into intracellular Ca2+ and cAMP signaling - PubMed (original) (raw)
Comparative Study
Cholangiocyte cilia detect changes in luminal fluid flow and transmit them into intracellular Ca2+ and cAMP signaling
Anatoliy I Masyuk et al. Gastroenterology. 2006 Sep.
Abstract
Background & aims: Cholangiocytes have primary cilia extending from the apical plasma membrane into the ductal lumen. While the physiologic significance of cholangiocyte cilia is unknown, studies in renal epithelia suggest that primary cilia possess sensory functions. Here, we tested the hypothesis that cholangiocyte cilia are sensory organelles that detect and transmit luminal bile flow stimuli into intracellular Ca2+ ([Ca2+]i) and adenosine 3',5'-cyclic monophosphate (cAMP) signaling.
Methods: Scanning electron microscopy, transmission electron microscopy, and immunofluorescent confocal microscopy of rat isolated intrahepatic bile duct units (IBDUs) were used to detect and characterize cholangiocyte cilia. The fluid flow-induced changes in Ca2+ and cAMP levels in cholangiocytes of microperfused IBDUs were detected by epifluorescence microscopy and a fluorescence assay, respectively.
Results: In microperfused IBDUs, luminal fluid flow induced an increase in [Ca2+]i and caused suppression of the forskolin-stimulated cAMP increase. The fluid flow-induced changes in [Ca2+]i and cAMP levels were significantly reduced or abolished when cilia were removed by chloral hydrate or when ciliary-associated proteins polycystin-1 (a mechanoreceptor), polycystin-2 (a Ca2+ channel), and the Ca2+-inhibitable adenylyl cyclase isoform 6 were individually down-regulated by small interfering RNAs.
Conclusions: Cholangiocyte cilia are sensory organelles containing polycystin-1, polycystin-2, and adenylyl cyclase isoform 6 through which luminal fluid flow affects both [Ca2+]i and cAMP signaling in the cell. The data suggest a new model for regulation of ductal bile secretion involving cholangiocyte cilia.
Figures
Figure 1
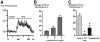
Primary cilia in rat intrahepatic bile ducts. TEM (A) and SEM (B, C) images of the primary cilium extending from the cholangiocyte apical plasma membrane. (A and B) A completely straight cilium projects into the lumen of an intrahepatic bile duct in the absence of fluid flow. (A) Structurally, the primary cilium consists of a microtubule-based axoneme covered by a specialized plasma membrane, and a basal body, which is derived from a mature mother centriole. The axoneme of a primary cilium has ‘9+0’ microtubule pattern (A, inset). (C) A SEM image of ciliary bending by luminal fluid flow moving in microperfused IBDUs from right to left. A cilium bent through an angle of approximately 60° (dashed lines) with respect to the axoneme axis and at position of 2.7 μm above the cell surface. Scale bar, 1 μm.
Figure 2
Luminal flow induces changes in [Ca2+]i in cholangiocytes of microperfused IBDUs. (A) A representative tracing of Fluo-4 fluorescence in response to luminal flow. (B) The maximal increase in Fluo-4 fluorescence was observed when IBDUs were exposed to an acute increase of luminal flow of 3 μl/sec. (C) The flow-induced increase in Fluo-4 fluorescence was suppressed in IBDUs perfused with Ca2+-free solutions or pretreated with thapsigargin. (n=6-8 IBDUs in each group, *, P<0.05).
Figure 3
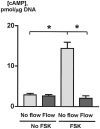
Luminal flow induces changes in cAMP in cholangiocytes of microperfused IBDUs. In the basal state (No FSK), cholangiocytes did not respond to luminal flow with changes in cAMP. In contrast, in forskolin-stimulated cholangiocytes (FSK) exposed to luminal fluid flow of 3 μl/sec, cAMP decreased to the basal levels (n=5-7 IBDUs in each group; *, P<0.001)
Figure 4
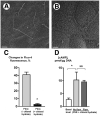
Chloral hydrate removes cholangiocyte cilia and abolishes the flow-induced changes in [Ca2+]i and cAMP.(A) An SEM image of the lumen of IBDUs incubated for 24 hours under control conditions shows normal primary cilia. (B) An SEM image of the lumen of IBDUs treated for 24 hours with 4 mM chloral hydrate shows the absence of normal primary cilia. (Bars 1 μm). (C) The flow-induced increase in Fluo-4 fluorescence [flow(-chloral hydrate)] was abolished by removal of cholangiocyte cilia by chloral hydrate [flow(+chloral hydrate)]. (D)Forskolin stimulated a 3.8 increase in cAMP level in cholangiocytes of non-perfused IBDU treated with chloral hydrate [No flow(FSK+chloral hydrate)] compared to the basal level of cAMP. No fluid flow-induced decrease in cAMP level was observed in microperfused IBDU stimulated with FSK and treated with chloral hydrate [Flow(FSK+chloral hydrate)]. (n=4-6 IBDUs in each group, *, P<0.05; NS indicates that the difference is not significant).
Figure 5
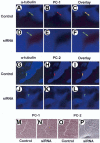
PC-1 and PC-2 in cholangiocyte cilia. Cholangiocytes of isolated IBDUs were stained with an antibody to a ciliary marker acetylated α-tubulin (green, A, D, G, J) and antibodies to PC-1 (red, B, E) and PC-2 (red, H, K). When the two images overlay co-localization of acetylated α-tubulin and PC-1 and PC-2 was seen (yellow, C, I) indicating that both polycystins are expressed in cholangiocyte cilia. In IBDUs incubated 24 hours with siRNAs against PC-1 (D-F) and PC-2 (J-L) no red staining (E, K) and overlay images in green (F, L) show that PC-1 and PC-2 do not present in cholangiocyte cilia after exposure of IBDUs to siRNAs. Nuclei were visualized by staining with DAPI (blue). The SEM images of control IBDUs (M, O) and IBDUs incubated with siRNAs against PC-1 (N) and PC-2 (P) show no difference in the morphology of cholangiocyte cilia.
Figure 6
PC-1 and PC-2 are involved in cholangiocyte responses to luminal flow. (A) An increase in Fluo-4 fluorescence in response to luminal fluid flow [No siRNAs and scrambled (Scr) siRNAs] was abolished by siRNAs to both, PC-1 and PC-2 suggesting the importance of a mechanoreceptor, PC-1, and a Ca2+ channel, PC-2, in the flow-induced increase in [Ca2+]i. (B, C) Forskolin stimulated an increase in cAMP levels in cholangiocytes of non-perfused IBDUs treated with scrambled siRNAs [No flow (control, FSK)] and siRNAs to PC-1 [No flow (PC-1 siRNA, FSK)] (B) and PC-2 [No flow (PC-2 siRNA, FSK)] (C) compared to the basal levels of cAMP. Luminal fluid flow induced a decrease in cAMP levels in cholangiocytes of microperfused IBDUs transfected with scrambled siRNAs [Flow (control, FSK)] but not with siRNAs to PC-1 [Flow (PC-1 siRNA, FSK)] and PC-2 [Flow (PC-2 siRNA, FSK)] suggesting the involvement of both PC-1 and PC-2 in the fluid flow-induced decrease in cAMP (n=3-5 IBDUs in each group, *, P<0.05; NS indicates that the difference is not significant).
Figure 7
Effects of luminal fluid flow on cAMP in cholangiocytes depend on extracellular Ca2+. Forskolin stimulated an increase in cAMP level in cholangiocytes of non-perfused IBDUs incubated in Ca2+-free KRB [No flow (Ca2+-free, FSK)] compared to the basal level of cAMP. No fluid flow-induced decrease in cAMP level was observed in IBDUs stimulated with FSK and perfused with Ca2+-free KRB [Flow (Ca2+-free, FSK)] suggesting the importance of extracellular Ca2+ in the fluid flow-induced suppression of FSK-stimulated cAMP signaling. (n=4-8 IBDUs in each group, *, P<0.001; NS indicates that the difference is not significant).
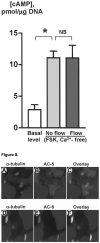
Figure 8
AC6 is expressed in cholangiocyte cilia. Cholangiocytes of isolated IBDUs were stained with an antibody to ciliary marker, acetylated a-tubulin (red) (A, D) and antibodies to AC5 and AC6 (green) (B, E). When the two images were overlay (C, F) co-localization of acetylated a-tubulin and AC6 (yellow, F) but not AC5 (red, C) was seen, indicating that Ca2+-inhibitable AC6 is expressed in cholangiocyte cilia. Nuclei were visualized by staining with DAPI (blue).
Figure 9
AC6 is involved in the fluid flow-induced decrease in cAMP. (A) Incubation of IBDUs for 24 hours with siRNAs to AC5 and AC6 resulted in significantly decreased levels of AC5 and AC6 mRNA compared to control IBDUs incubated with scrambled siRNAs. (B, C) Forskolin stimulated an increase in cAMP levels in cholangiocytes of non-perfused IBDUs treated with scrambled siRNAs [No flow (control, FSK)] and siRNAs to AC5 [No flow (AC5 siRNA, FSK)] (B) and AC6 [No flow (AC6 siRNA, FSK]) (C) compared to the basal levels of cAMP. Luminal fluid flow induced a decrease in cAMP levels in cholangiocytes of microperfused IBDUs transfected with scrambled siRNAs [Flow(control, FSK)] and with siRNAs to AC5 [Flow (AC5 siRNA, FSK)] but not with siRNA to AC6 [Flow (AC6 siRNA, FSK)] suggesting the involvement of AC6 in the fluid flow-induced decrease in cAMP (n=4-8 IBDUs in each group, *, P< 0.05; NS indicates that the difference is not significant).
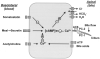
Figure 10
Working model of coordinated regulation of cholangiocyte secretion. Cholangiocytes possess numerous transporters, exchangers and channels necessary for ductal bile formation. The functions of these proteins on the apical plasma membrane are regulated by different regulatory molecules via specific receptors and the cAMP and [Ca2+]i signaling pathways. Secretin induces cholangiocyte bicarbonate rich fluid secretion via the cAMP-PKA signaling pathway by activation of apical CFTR Cl- channel resulting in extrusion of Cl ions, which in turn stimulate Cl-/HCO-3 exchanger and subsequent secretion of HCO-3. Secreted bicarbonate ions drive passive AQP1-mediated water transport in response to established osmotic gradients. Secretin-stimulated cholangiocyte secretion may be potentiated or inhibited by different regulatory molecules (e.g., somatostatin, acetylcholine, nucleotides, bile acids, etc). Primary cilia located on the apical plasma membrane may represent a novel regulatory mechanism that is involved in coordinated regulation of cholangiocyte secretion (see text for details). +, activation; -, inhibition.
Similar articles
- Cholangiocyte cilia express TRPV4 and detect changes in luminal tonicity inducing bicarbonate secretion.
Gradilone SA, Masyuk AI, Splinter PL, Banales JM, Huang BQ, Tietz PS, Masyuk TV, Larusso NF. Gradilone SA, et al. Proc Natl Acad Sci U S A. 2007 Nov 27;104(48):19138-43. doi: 10.1073/pnas.0705964104. Epub 2007 Nov 16. Proc Natl Acad Sci U S A. 2007. PMID: 18024594 Free PMC article. - Cholangiocyte primary cilia are chemosensory organelles that detect biliary nucleotides via P2Y12 purinergic receptors.
Masyuk AI, Gradilone SA, Banales JM, Huang BQ, Masyuk TV, Lee SO, Splinter PL, Stroope AJ, Larusso NF. Masyuk AI, et al. Am J Physiol Gastrointest Liver Physiol. 2008 Oct;295(4):G725-34. doi: 10.1152/ajpgi.90265.2008. Epub 2008 Aug 7. Am J Physiol Gastrointest Liver Physiol. 2008. PMID: 18687752 Free PMC article. - Defects in cholangiocyte fibrocystin expression and ciliary structure in the PCK rat.
Masyuk TV, Huang BQ, Ward CJ, Masyuk AI, Yuan D, Splinter PL, Punyashthiti R, Ritman EL, Torres VE, Harris PC, LaRusso NF. Masyuk TV, et al. Gastroenterology. 2003 Nov;125(5):1303-10. doi: 10.1016/j.gastro.2003.09.001. Gastroenterology. 2003. PMID: 14598246 - Cholangiocyte primary cilia in liver health and disease.
Masyuk AI, Masyuk TV, LaRusso NF. Masyuk AI, et al. Dev Dyn. 2008 Aug;237(8):2007-12. doi: 10.1002/dvdy.21530. Dev Dyn. 2008. PMID: 18407555 Free PMC article. Review. - The vertebrate primary cilium is a sensory organelle.
Pazour GJ, Witman GB. Pazour GJ, et al. Curr Opin Cell Biol. 2003 Feb;15(1):105-10. doi: 10.1016/s0955-0674(02)00012-1. Curr Opin Cell Biol. 2003. PMID: 12517711 Review.
Cited by
- Physiology of cholangiocytes.
Tabibian JH, Masyuk AI, Masyuk TV, O'Hara SP, LaRusso NF. Tabibian JH, et al. Compr Physiol. 2013 Jan;3(1):541-65. doi: 10.1002/cphy.c120019. Compr Physiol. 2013. PMID: 23720296 Free PMC article. Review. - MicroRNAs in biliary diseases.
Munoz-Garrido P, García-Fernández de Barrena M, Hijona E, Carracedo M, Marín JJ, Bujanda L, Banales JM. Munoz-Garrido P, et al. World J Gastroenterol. 2012 Nov 21;18(43):6189-96. doi: 10.3748/wjg.v18.i43.6189. World J Gastroenterol. 2012. PMID: 23180938 Free PMC article. Review. - Primary cilia-mediated mechanotransduction in human mesenchymal stem cells.
Hoey DA, Tormey S, Ramcharan S, O'Brien FJ, Jacobs CR. Hoey DA, et al. Stem Cells. 2012 Nov;30(11):2561-70. doi: 10.1002/stem.1235. Stem Cells. 2012. PMID: 22969057 Free PMC article. - The emerging role of Arf/Arl small GTPases in cilia and ciliopathies.
Li Y, Ling K, Hu J. Li Y, et al. J Cell Biochem. 2012 Jul;113(7):2201-7. doi: 10.1002/jcb.24116. J Cell Biochem. 2012. PMID: 22389062 Free PMC article. Review. - Cholangiocyte cilia are abnormal in syndromic and non-syndromic biliary atresia.
Chu AS, Russo PA, Wells RG. Chu AS, et al. Mod Pathol. 2012 May;25(5):751-7. doi: 10.1038/modpathol.2011.212. Epub 2012 Feb 3. Mod Pathol. 2012. PMID: 22301700 Free PMC article.
References
- Wheatley DN. Primary cilia in normal and pathological tissues. Pathobiology. 1995;63:222–238. - PubMed
- Praetorius HA, Spring KR. A physiological view of the primary cilium. Ann Rev Physiol. 2005;67:515–529. - PubMed
- Davenport JR, Yoder BK. An incredible decade for the primary cilium: a look at a once-forgotten organelle. Am J Physiol Renal Physiol. 2005;289:F1159–F1169. - PubMed
- McGrath J, Somlo S, Makova S, Tian X, Brueckner M. Two populations of node monocilia initiate left-right asymmetry in the mouse. Cell. 2003;114:61–73. - PubMed
- Yost HJ. Left-right asymmetry: nodal cilia make and catch a wave. Curr Biol. 2003;13:R808–R809. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous