Three-dimensional reconstruction of the membrane skeleton at the plasma membrane interface by electron tomography - PubMed (original) (raw)
Three-dimensional reconstruction of the membrane skeleton at the plasma membrane interface by electron tomography
Nobuhiro Morone et al. J Cell Biol. 2006.
Abstract
Three-dimensional images of the undercoat structure on the cytoplasmic surface of the upper cell membrane of normal rat kidney fibroblast (NRK) cells and fetal rat skin keratinocytes were reconstructed by electron tomography, with 0.85-nm-thick consecutive sections made approximately 100 nm from the cytoplasmic surface using rapidly frozen, deeply etched, platinum-replicated plasma membranes. The membrane skeleton (MSK) primarily consists of actin filaments and associated proteins. The MSK covers the entire cytoplasmic surface and is closely linked to clathrin-coated pits and caveolae. The actin filaments that are closely apposed to the cytoplasmic surface of the plasma membrane (within 10.2 nm) are likely to form the boundaries of the membrane compartments responsible for the temporary confinement of membrane molecules, thus partitioning the plasma membrane with regard to their lateral diffusion. The distribution of the MSK mesh size as determined by electron tomography and that of the compartment size as determined from high speed single-particle tracking of phospholipid diffusion agree well in both cell types, supporting the MSK fence and MSK-anchored protein picket models.
Figures
Figure 1.
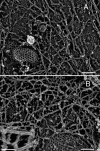
A bird's-eye view of the large cytoplasmic surface of the upper cell membrane (the membrane facing the buffer rather than the coverslip) of an NRK cell observed by rapid-freeze, deep-etch, freeze-replica EM. Bar, 2.5 μm.
Figure 2.
Magnified MSK images of an NRK and FRSK cell on the cytoplasmic surface of the upper membrane. (A) NRK cell; (B) FRSK cell. Clathrin- coated structures (A and B) and a caveola (A) show the cytoplasmic surface. The striped banding patterns with the 5.5-nm periodicity on individual filaments are characteristic of actin filaments. These images also reveal close links of the MSK actin filaments with the clathrin-coated structures and caveolae. Bars (A and B), 100 nm; (inset) 50 nm.
Figure 3.
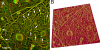
Immunogold labeling also indicates that the major component of MSK is actin filaments (NRK cell). Actin filaments were indirectly immunolabeled with 5-nm colloidal gold particles coated with secondary antibodies. Each gold particle can be identified as a clear white spot (colloidal gold particle) surrounded by a fuzzy gray ring, which is caused by the platinum rotary shadowing around the secondary antibody coating of the gold particle. Representative probe images are indicated by arrowheads. (A) Most of the filamentous structures are labeled by colloidal gold probes. (B and C) The boxed area in A is shown at a higher magnification. In C, the gold particles are marked by yellow dots. The filaments with the 5.5-nm striped banding pattern, which is characteristic of the actin filament, are labeled with these gold probes. Bars (A), 200 nm; (B and C) 50 nm.
Figure 4.
Stereo electron micrographs and 3D reconstructed images of the undercoat structure, CCPs, and caveolae in NRK cells. (A) An EM anaglyph of the undercoat structure generated at ±12° of the tilt angle among the 131 tilt images (acquired in the range of ±65° with 1° steps). Use view glasses for the 3D structure (left = red). See Video 1 for all 131 of the tilt images. Arrows indicate actin filaments protruding from the membrane cytoplasmic surface toward the cytoplasm. The arrows point to the places where the protruding actin filaments intersect with the MSK meshwork located close to the membrane. (B) The 3D undercoat structure reconstructed from the tilt images shown in Video 1. See Video 2, where the 3D structure is rotated (available at
http://www.jcb.org/cgi/content/full/jcb.200606007/DC1
). Bar, 100 nm.
Figure 5.
A series of sliced images of the actin MSK on the plasma membrane cytoplasmic surface of an NRK cell. (A) A typical actin MSK structure used for analysis of the mesh size on the cytoplasmic surface of the plasma membrane using computed tomography. An anaglyph of the undercoat structure generated at ±12° of the tilt angle among the 97 tilt images (acquired in the range of ±48° with 1° steps). See Video 3 for all 97 of the tilt images. (B) 10 consecutive sections, each 0.85-nm thick, are superimposed, and six of these superimposed images, which represent 60 image sections out of 121 image sections, are shown from the cytoplasmic side toward the plasma membrane side. The numbers here indicate the number of slices counted from the cytoplasmic side. The actin-based MSK near the cytoplasmic surface of the plasma membrane is visible on images 81–110. All 121 of the sliced images of every 0.85 nm are shown in Video 4 (available at
http://www.jcb.org/cgi/content/full/jcb.200606007/DC1
).
Figure 6.
The method for determining the MSK mesh on the cytoplasmic surface of the plasma membrane, which possibly delimits the compartments of the plasma membrane, using the 3D reconstructed images of the MSK (an NRK cell). (A and B) The images on the far left are the ∼0–6.8- or ∼6.8–13.6-nm sections, each of which is a stack of eight 0.85-nm sections of 670 × 670 nm. These are from a series of 121 image sections (0.85-nm thick) from the cytoplasmic surface after the tilt and long wavelength undulation of the cell surface were corrected. The boxed areas in A and B (330 × 330 nm) are expanded on the right of these image stacks, with a section thickness of 1.7 nm (two 0.85-nm sections are superimposed; 330 × 330 nm for each image). (C) The outline of each actin filament adjacent to the membrane surface (green, which could not be observed above 10.2 nm) and that of each actin filament that could be observed above 10.2 nm (red). The same view field and magnification as those for the thinner sections shown in A and B (330 × 330 nm). See Materials and methods for details. (D) The outline of actin filaments in a greater view field, which is the same as those in the thick sections (∼0–6.8 and ∼6.8–13.6 nm) in A and B (670 × 670 nm, expanded here). (E) The image of the ∼0–6.8-nm sections (670 × 670 nm) superimposed by the image of areas surrounded by the filaments outlined in green in D (green areas with yellow outlines). According to the fence and picket models, these areas are likely to be the compartments where membrane molecules are temporarily confined.
Figure 7.
The MSK meshwork directly on the cytoplasmic surface of the plasma membrane. The central parts of the figures in the top row (bar, 300 nm) are magnified by a factor of three and are shown in the bottom row (bar, 100 nm). (A) Typical stereo views of the plasma membrane specimen (anaglyph; left = red). (B) Normal electron micrographs of the plasma membrane samples. The same view fields as those in A. (C) The areas delimited by the actin filaments closely apposed to the cytoplasmic surface of the cell membrane are shown. Different colors are shown to help the eye.
Figure 8.
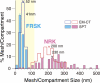
Comparison of the distributions of the MSK mesh size on the cytoplasmic surface of the plasma membrane estimated by electron tomography with that of the compartment size determined from the phospholipid diffusion data for NRK and FRSK cells. Electron tomography, open bars; phospholipid diffusion data, closed bars (adapted from Fujiwara et al., 2002 and Murase et al., 2004). NRK, pink; FRSK, blue. Within the same cell type, the MSK mesh size and diffusion compartment size exhibited similar distributions (compare the open and closed bars with the same color). The actual sizes are quite different between NRK and FRSK cells. EM-CT, EM-based computer tomograph; SPT, single-particle tracking.
Similar articles
- Freeze-etch electron tomography for the plasma membrane interface.
Morone N. Morone N. Methods Mol Biol. 2010;657:275-86. doi: 10.1007/978-1-60761-783-9_22. Methods Mol Biol. 2010. PMID: 20602224 - Adhesion structures and their cytoskeleton-membrane interactions at podosomes of osteoclasts in culture.
Akisaka T, Yoshida H, Suzuki R, Takama K. Akisaka T, et al. Cell Tissue Res. 2008 Mar;331(3):625-41. doi: 10.1007/s00441-007-0552-x. Epub 2007 Dec 18. Cell Tissue Res. 2008. PMID: 18087726 - Confined diffusion of transmembrane proteins and lipids induced by the same actin meshwork lining the plasma membrane.
Fujiwara TK, Iwasawa K, Kalay Z, Tsunoyama TA, Watanabe Y, Umemura YM, Murakoshi H, Suzuki KG, Nemoto YL, Morone N, Kusumi A. Fujiwara TK, et al. Mol Biol Cell. 2016 Apr 1;27(7):1101-19. doi: 10.1091/mbc.E15-04-0186. Epub 2016 Feb 10. Mol Biol Cell. 2016. PMID: 26864625 Free PMC article. - Paradigm shift of the plasma membrane concept from the two-dimensional continuum fluid to the partitioned fluid: high-speed single-molecule tracking of membrane molecules.
Kusumi A, Nakada C, Ritchie K, Murase K, Suzuki K, Murakoshi H, Kasai RS, Kondo J, Fujiwara T. Kusumi A, et al. Annu Rev Biophys Biomol Struct. 2005;34:351-78. doi: 10.1146/annurev.biophys.34.040204.144637. Annu Rev Biophys Biomol Struct. 2005. PMID: 15869394 Review. - Three-dimensional molecular architecture of the plasma-membrane-associated cytoskeleton as reconstructed by freeze-etch electron tomography.
Morone N, Nakada C, Umemura Y, Usukura J, Kusumi A. Morone N, et al. Methods Cell Biol. 2008;88:207-36. doi: 10.1016/S0091-679X(08)00412-3. Methods Cell Biol. 2008. PMID: 18617036 Review. No abstract available.
Cited by
- Statin-induced increase in actin polymerization modulates GPCR dynamics and compartmentalization.
Sarkar P, Chattopadhyay A. Sarkar P, et al. Biophys J. 2023 Jun 6;122(11):1938-1955. doi: 10.1016/j.bpj.2022.08.039. Epub 2022 Aug 30. Biophys J. 2023. PMID: 36045572 Free PMC article. - Analysis of turnover dynamics of the submembranous actin cortex.
Fritzsche M, Lewalle A, Duke T, Kruse K, Charras G. Fritzsche M, et al. Mol Biol Cell. 2013 Mar;24(6):757-67. doi: 10.1091/mbc.E12-06-0485. Epub 2013 Jan 23. Mol Biol Cell. 2013. PMID: 23345594 Free PMC article. - Flow Arrest in the Plasma Membrane.
Chein M, Perlson E, Roichman Y. Chein M, et al. Biophys J. 2019 Sep 3;117(5):810-816. doi: 10.1016/j.bpj.2019.07.001. Epub 2019 Jul 5. Biophys J. 2019. PMID: 31326106 Free PMC article. - Cortical Actin Dynamics in Endothelial Permeability.
Belvitch P, Htwe YM, Brown ME, Dudek S. Belvitch P, et al. Curr Top Membr. 2018;82:141-195. doi: 10.1016/bs.ctm.2018.09.003. Epub 2018 Oct 15. Curr Top Membr. 2018. PMID: 30360779 Free PMC article. - Single-particle tracking of membrane protein diffusion in a potential: simulation, detection, and application to confined diffusion of CFTR Cl- channels.
Jin S, Haggie PM, Verkman AS. Jin S, et al. Biophys J. 2007 Aug 1;93(3):1079-88. doi: 10.1529/biophysj.106.102244. Epub 2007 May 4. Biophys J. 2007. PMID: 17483157 Free PMC article.
References
- Bennett, V. 1990. Spectrin-based membrane skeleton: a multipotential adaptor between plasma membrane and cytoplasm. Physiol. Rev. 70:1029–1065. - PubMed
- Bennett, V., and L. Chen. 2001. Ankyrins and cellular targeting of divers membrane proteins to physiological sites. Curr. Opin. Cell Biol. 13:61–67. - PubMed
- Branton, D., C.M. Cohen, and J. Tyler. 1981. Interaction of cytoskeletal proteins on the human erythrocyte membrane. Cell. 24:24–32. - PubMed
- Bussell, S.J., D.A. Hammer, and D.L. Koch. 1994. The effect of hydrodynamic interactions on the tracer and gradient diffusion of integral membrane-proteins in lipid bilayers. J. Fluid Mech. 258:167–190.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources