Immunoprivileged status of the liver is controlled by Toll-like receptor 3 signaling - PubMed (original) (raw)
. 2006 Sep;116(9):2456-63.
doi: 10.1172/JCI28349.
Panco Georgiev, Mike Recher, Alexander A Navarini, Andreas Bergthaler, Mathias Heikenwalder, Nicola L Harris, Tobias Junt, Bernhard Odermatt, Pierre-Alain Clavien, Hanspeter Pircher, Shizuo Akira, Hans Hengartner, Rolf M Zinkernagel
Affiliations
- PMID: 16955143
- PMCID: PMC1555644
- DOI: 10.1172/JCI28349
Immunoprivileged status of the liver is controlled by Toll-like receptor 3 signaling
Karl S Lang et al. J Clin Invest. 2006 Sep.
Abstract
The liver is known to be a classical immunoprivileged site with a relatively high resistance against immune responses. Here we demonstrate that highly activated liver-specific effector CD8+ T cells alone were not sufficient to trigger immune destruction of the liver in mice. Only additional innate immune signals orchestrated by TLR3 provoked liver damage. While TLR3 activation did not directly alter liver-specific CD8+ T cell function, it induced IFN-alpha and TNF-alpha release. These cytokines generated expression of the chemokine CXCL9 in the liver, thereby enhancing CD8+ T cell infiltration and liver disease in mice. Thus, nonspecific activation of innate immunity can drastically enhance susceptibility to immune destruction of a solid organ.
Figures
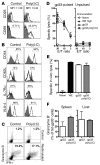
Figure 1. TLR3 ligation converts autoreactivity into autoimmune disease.
107 splenocytes from LCMV-gp33/H-2Db–specific TCR-Tg 318 mice were injected i.v. into naive Alb-1 mice or control C57BL/6 mice on day –1. (A) Seven days after transfer, mice were analyzed for gp33-specific T cells and for liver enzymes. Proliferation of transferred cells was analyzed with CFSE (Supplemental Figure 1). (B) After transfer of 318 splenocytes, Alb-1 mice were infected with 200 PFU LCMV-WE on day 0 (filled circles), and numbers of tet-gp33+ CD8+ T cells determined in peripheral blood over time. Serum activities of ALT and bilirubin serum concentrations were also determined (n = 5). *P > 0.05. (C) Mice were immunized with gp33 (1 mg in PBS) and CpG on days 0 and 4 (open squares). A separate group of mice was additionally treated with poly(I:C) on day 7 (arrows) (shaded squares). Numbers of blood tet-gp33+CD8+ T cells, serum ALT activities, and bilirubin serum concentrations were determined (n = 5–7 per group).
Figure 2. No influence of TLR3 ligation on CD8+ T cell effector function.
107 splenocytes from LCMV-gp33/H-2Db–specific TCR-Tg 318 mice were injected i.v. into naive C57BL/6 mice. Mice were immunized with gp33 (1 mg in PBS) and CpG on days 0 and 4. A separate group of mice was additionally treated with poly(I:C) on day 7. The phenotype and function of CD8+ T cells were analyzed 24 hours (A–E; n = 3 per group) or 12 hours (F; n = 2–3 per group) later. (A) Splenocytes were analyzed by FACS for early activation marker CD25 and CD69. Histogram plots show cells gated on CD8 and Thy1.1 (marker for 318 T cells). Gray shading indicates staining with isotype control antibody. Values for FACS analysis give mean fluorecence intensity (MFI). For other plots in A, B, and C, the number of positive expressing cells (marked by bar or quadrant) are given. (B) Splenocytes were further analyzed for surface expression of CD44, CD62L, IL-7Rα, and VLA-4 (CD49d). Histogram plots show cells gated for CD8 expression and tet-gp33 expression (indicated by a black line) or tet-gp33–negative cells (control expression, indicated by gray shading). (C) Splenocytes were restimulated in vitro with or without gp33, then analyzed for intracellular expression of granzyme B and IFN-γ. Dot plots show cells gated for CD8 expression. (D and E) Splenocytes were analyzed for their ability to lyse peptide-loaded target cells in vitro (D) or in vivo (E). Naive splenocytes served as negative control; splenocytes from mice infected with 2 × 106 pfu of LCMV-WE (WE high) served as positive control. (F) Eight hours after treatment with poly(I:C), splenocytes were analyzed for their expression of granzyme B (n = 2–3).
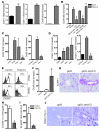
Figure 3. Activation of TLR3 and regulation of Icam-1, Vcam-1, and Cxcl9.
(A–E) Mice were treated with poly(I:C). After 24 hours, RT-PCR analysis was performed in livers from C57BL/6 mice (n = 3) for Icam-1, Vcam-1, and Cxcl9 (A); Cxcl9 in livers from BALB/c and Tlr3–/– mice and Tlr3–/–_→BALB/c and BALB/c→_Tlr3–/– bone marrow chimeras (B); Cxcl9 (C) or Vcam-1 (D) in livers from Ifnar–/–, Ifngr–/–, and Tnfr1–/– mice plus corresponding wild-type controls (n = 3). (E) Eight hours after poly(I:C) treatment, blood and spleen cells of C57BL/6 mice were analyzed for surface expression of CXCR3 by flow cytometry. Histogram plots show CD8+ T cells gated for low CD44 expression (naive CD8+ T cells, gray shaded area) or high CD44 expression (memory CD8+ T cells, black line; 15%–20% of all CD8+ T cells). (F–J) 107 splenocytes from LCMV-gp33/H-2Db–specific TCR-Tg 318 mice were injected i.v. into C57BL/6 mice (F–I) or Alb-1 mice (J) on day –1. Recipients were then immunized with gp33 (1 mg in PBS) together with CpG on days 0 and 4. One group of mice was additionally treated with poly(I:C) on day 7. On day 8, livers were analyzed for Cxcl9 by RT-PCR (F) or VCAM-1 by histology (magnification, ×200) (G). Ten hours after poly(I:C) treatment, mice were analyzed for expression of CXCR3 on tet-gp33+ CD8+ splenocytes (H) and for absolute numbers of tet-gp33+ CD8+ T cells in the blood (I). (J) Livers of immunized Alb-1 mice were analyzed for the presence of autoreactive T cells using anti-CD90.1/Thy1.1 antibody (magnification, ×50, ×200). *P < 0.05.
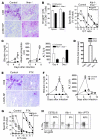
Figure 4. Homing is crucial for LCMV-induced autoimmune hepatitis.
(A–C) 107 splenocytes from LCMV-gp33/H-2Db–specific TCR-Tg 318 mice were transferred i.v. into Alb-1+ Ifnar–/– mice (C57BL/6 background) and littermate controls (Alb-1+ Ifnar+/– or Alb-1+ Ifnar+/+) on day –1. Recipients were immunized with 200 pfu LCMV on day 0. (A) On day 7, Alb-1+ Ifnar–/– mice died, and livers were analyzed for CD8+ T cell infiltration and LCMV nucleoprotein (LCMV-NP) by immunohistology (magnification, ×100). (B) CD8+ T cells were analyzed by tetramer staining and in an in vitro CTL assay. (C) Serum was analyzed for bilirubin concentrations, alkaline phosphatase activity (a marker for bile cholestasis), and ALT activity (n = 3 per group). (D) _Ifnar–/–_mice (Sv129 background without transfer of 318 T cells) and corresponding control mice were infected with 200 pfu LCMV-WE. After 7 days, livers were analyzed for expression of CXCL9. *P > 0.05. (E–H) 107 splenocytes from LCMV-gp33/H-2Db–specific TCR-Tg 318 mice were transferred i.v. into naive Alb-1 mice or C57BL/6 mice on day –1. Recipients were then infected with 200 PFU LCMV-WE on day 0. One group was further treated with 0.5 μg of PTX on days 0 and 3. (E) On day 6, liver sections were analyzed for CD8+ T cell infiltration or for viral antigen (LCMV-NP) by histology (magnification, ×100; n = 2 per group). Serum was analyzed for bilirubin concentration and ALT activity (n = 4 per group) (F), and CD8+ T cell effector function was assessed by an in vitro CTL assay (G). (H) Autoreactive CD8+ T cells and their IL-7Rα expression were analyzed in the blood (n = 4 per group).
Comment in
- Toll-like receptors and IFN-alpha: partners in autoimmunity.
Colonna M. Colonna M. J Clin Invest. 2006 Sep;116(9):2319-22. doi: 10.1172/JCI29879. J Clin Invest. 2006. PMID: 16955132 Free PMC article. - Toll-like receptor-3 and the regulation of intrahepatic immunity: implications for interferon-alpha therapy.
Bertolino P, Holz LE. Bertolino P, et al. Hepatology. 2007 Jan;45(1):250-1. doi: 10.1002/hep.21504. Hepatology. 2007. PMID: 17187430
Similar articles
- Toll-like receptor-3 and the regulation of intrahepatic immunity: implications for interferon-alpha therapy.
Bertolino P, Holz LE. Bertolino P, et al. Hepatology. 2007 Jan;45(1):250-1. doi: 10.1002/hep.21504. Hepatology. 2007. PMID: 17187430 - Peptide vaccination of mice immune to LCMV or vaccinia virus causes serious CD8 T cell-mediated, TNF-dependent immunopathology.
Liu F, Feuer R, Hassett DE, Whitton JL. Liu F, et al. J Clin Invest. 2006 Feb;116(2):465-75. doi: 10.1172/JCI25608. Epub 2006 Jan 19. J Clin Invest. 2006. PMID: 16424939 Free PMC article. - Interleukin-1 receptor-associated kinase-1 plays an essential role for Toll-like receptor (TLR)7- and TLR9-mediated interferon-{alpha} induction.
Uematsu S, Sato S, Yamamoto M, Hirotani T, Kato H, Takeshita F, Matsuda M, Coban C, Ishii KJ, Kawai T, Takeuchi O, Akira S. Uematsu S, et al. J Exp Med. 2005 Mar 21;201(6):915-23. doi: 10.1084/jem.20042372. Epub 2005 Mar 14. J Exp Med. 2005. PMID: 15767370 Free PMC article. - TLR3-mediated IFN-β gene induction is negatively regulated by the TLR adaptor MyD88 adaptor-like.
Siednienko J, Halle A, Nagpal K, Golenbock DT, Miggin SM. Siednienko J, et al. Eur J Immunol. 2010 Nov;40(11):3150-60. doi: 10.1002/eji.201040547. Epub 2010 Oct 19. Eur J Immunol. 2010. PMID: 20957750 - TLR3-Mediated CD8+ Dendritic Cell Activation Is Coupled with Establishment of a Cell-Intrinsic Antiviral State.
Széles L, Meissner F, Dunand-Sauthier I, Thelemann C, Hersch M, Singovski S, Haller S, Gobet F, Fuertes Marraco SA, Mann M, Garcin D, Acha-Orbea H, Reith W. Széles L, et al. J Immunol. 2015 Aug 1;195(3):1025-33. doi: 10.4049/jimmunol.1402033. Epub 2015 Jun 22. J Immunol. 2015. PMID: 26101320
Cited by
- Tumor restriction by type I collagen opposes tumor-promoting effects of cancer-associated fibroblasts.
Bhattacharjee S, Hamberger F, Ravichandra A, Miller M, Nair A, Affo S, Filliol A, Chin L, Savage TM, Yin D, Wirsik NM, Mehal A, Arpaia N, Seki E, Mack M, Zhu D, Sims PA, Kalluri R, Stanger BZ, Olive KP, Schmidt T, Wells RG, Mederacke I, Schwabe RF. Bhattacharjee S, et al. J Clin Invest. 2021 Jun 1;131(11):e146987. doi: 10.1172/JCI146987. J Clin Invest. 2021. PMID: 33905375 Free PMC article. - Type I interferon receptor deficiency prevents murine Sjogren's syndrome.
Szczerba BM, Rybakowska PD, Dey P, Payerhin KM, Peck AB, Bagavant H, Deshmukh US. Szczerba BM, et al. J Dent Res. 2013 May;92(5):444-9. doi: 10.1177/0022034513483315. Epub 2013 Mar 26. J Dent Res. 2013. PMID: 23533183 Free PMC article. - Toll like receptor 3 plays a critical role in the progression and severity of acetaminophen-induced hepatotoxicity.
Cavassani KA, Moreira AP, Habiel D, Ito T, Coelho AL, Allen RM, Hu B, Raphelson J, Carson WF 4th, Schaller MA, Lukacs NW, Omary MB, Hogaboam CM, Kunkel SL. Cavassani KA, et al. PLoS One. 2013 Jun 7;8(6):e65899. doi: 10.1371/journal.pone.0065899. Print 2013. PLoS One. 2013. PMID: 23762449 Free PMC article. - Systemic administration of TLR3 agonist induces IL-7 expression and IL-7-dependent CXCR3 ligand production in the lung.
Jin JO, Yu Q. Jin JO, et al. J Leukoc Biol. 2013 Mar;93(3):413-25. doi: 10.1189/jlb.0712360. Epub 2012 Dec 27. J Leukoc Biol. 2013. PMID: 23271706 Free PMC article. - Autoimmunity stimulated by adoptively transferred dendritic cells is initiated by both alphabeta and gammadelta T cells but does not require MyD88 signaling.
Martin DA, Zhang K, Kenkel J, Hughes G, Clark E, Davidson A, Elkon KB. Martin DA, et al. J Immunol. 2007 Nov 1;179(9):5819-28. doi: 10.4049/jimmunol.179.9.5819. J Immunol. 2007. PMID: 17947655 Free PMC article.
References
- Kamada N., Davies H.S., Roser B. Reversal of transplantation immunity by liver grafting. Nature. 1981;292:840–842. - PubMed
- Bumgardner G.L., Orosz C.G. Unusual patterns of alloimmunity evoked by allogeneic liver parenchymal cells. Immunol. Rev. 2000;174:260–279. - PubMed
- Diamantis I., Boumpas D.T. Autoimmune hepatitis: evolving concepts. Autoimmun. Rev. 2004;3:207–214. - PubMed
- Okano N., et al. Clinicopathological features of acute-onset autoimmune hepatitis. Hepatol. Res. 2003;25:263–270. - PubMed
- Luxon B.A. Autoimmune hepatitis. Making sense of all those antibodies. Postgrad. Med. 2003;114:79–82, 85–88. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials