The role of G-protein-coupled receptor kinase 5 in pathogenesis of sporadic Parkinson's disease - PubMed (original) (raw)
. 2006 Sep 6;26(36):9227-38.
doi: 10.1523/JNEUROSCI.0341-06.2006.
Manabu Wada, Saori Goto, Hiroki Karube, Masahiro Sakamoto, Chang-Hong Ren, Shingo Koyama, Hikaru Nagasawa, Hideki Kimura, Toru Kawanami, Keiji Kurita, Katsushi Tajima, Makoto Daimon, Masanori Baba, Takashi Kido, Sachiko Saino, Kaoru Goto, Hironobu Asao, Chihumi Kitanaka, Emi Takashita, Seiji Hongo, Takao Nakamura, Takamasa Kayama, Yoshihiro Suzuki, Kazuo Kobayashi, Tadashi Katagiri, Katsuro Kurokawa, Masayuki Kurimura, Itaru Toyoshima, Kazuhiro Niizato, Kuniaki Tsuchiya, Takeshi Iwatsubo, Masaaki Muramatsu, Hiroto Matsumine, Takeo Kato
Affiliations
- PMID: 16957079
- PMCID: PMC6674490
- DOI: 10.1523/JNEUROSCI.0341-06.2006
The role of G-protein-coupled receptor kinase 5 in pathogenesis of sporadic Parkinson's disease
Shigeki Arawaka et al. J Neurosci. 2006.
Abstract
Sporadic Parkinson's disease (sPD) is a common neurodegenerative disorder, characterized by selective degeneration of dopaminergic neurons in the substantia nigra. Although the pathogenesis of the disease remains undetermined, phosphorylation of alpha-synuclein and its oligomer formation seem to play a key role. However, the protein kinase(s) involved in the phosphorylation in the pathogenesis of sPD has not been identified. Here, we found that G-protein-coupled receptor kinase 5 (GRK5) accumulated in Lewy bodies and colocalized with alpha-synuclein in the pathological structures of the brains of sPD patients. In cotransfected cells, GRK5 phosphorylated Ser-129 of alpha-synuclein at the plasma membrane and induced translocation of phosphorylated alpha-synuclein to the perikaryal area. GRK5-catalyzed phosphorylation also promoted the formation of soluble oligomers and aggregates of alpha-synuclein. Genetic association study revealed haplotypic association of the GRK5 gene with susceptibility to sPD. The haplotype contained two functional single-nucleotide polymorphisms, m22.1 and m24, in introns of the GRK5 gene, which bound to YY1 (Yin Yang-1) and CREB-1 (cAMP response element-binding protein 1), respectively, and increased transcriptional activity of the reporter gene. The results suggest that phosphorylation of alpha-synuclein by GRK5 plays a crucial role in the pathogenesis of sPD.
Figures
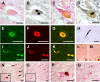
Figure 1.
Anti-GRK5 antibody immunostains Lewy bodies in the brainstem of sPD patients. A, B, The same section of the locus ceruleus stained with H&E (A) and anti-GRK5 (H-64) (B). C, D, Pale bodies in the locus ceruleus (C, H&E; D, H-64) were also positive for GRK5 staining. E–G, Confocal microscopic images of Lewy body in a nigral neuron using H-64 (E) and anti-αS antibodies (LB509; F). A merged image of E and F is shown in G. H, Filamentous or rod-like structure in the substantia nigra stained with H-64. I–K, Confocal microscopic images of filamentous or rod-like structures in the substantia nigra using H-64 (I) and LB509 (J). A merged image of I and J is shown in K. L, M, No immunostaining for GRK5 was found in cortical-type Lewy bodies (L, LB509; M, H-64). N–Q, The immunostaining with H-64 (N, P) was completely abolished by the addition of recombinant human GRK5 to the primary antibody solution (O, Q). The boxed areas in N and O are shown in P and Q, respectively. The arrows in N indicate Lewy bodies. L–O are the serial sections, and the asterisks indicate the same blood vessels. L–Q are counterstained with neutral fast red. Scale bars, 20 μm.
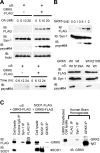
Figure 2.
GRK5 phosphorylates αS at Ser-129. A, Immunoblots (IB) using one of the antibodies for GRK5–FLAG (FLAG), total αS (Syn-1), pS129–αS (psyn#64), and β-actin (AC-15). HEK293–αS cells transfected with or without GRK5–FLAG cDNA were treated with OA at concentrations of 0, 5, 10, and 20 n
m
(top). They were incubated for 0, 6, 12, and 24 h in the presence of 20 n
m
OA in medium (bottom). In HEK293–αS cells expressing GRK5, pS129–αS was detected in OA dose- and incubation time-dependent manners. The levels of total αS and actin were constant (top). B, Phosphorylation of αS at Ser-129 through GRK5 enzymatic activity. Top, HEK293–αS cells were transfected with increasing amounts of GRK5–FLAG cDNA, and cell lysates were subjected to immunoblot analysis. Although the expression levels of total αS were approximately equal, the pS129–αS levels increased in parallel to the level of GRK5. Bottom, Immunoblotting of cell lysates expressing the combination of wild-type (Wt) αS or αS–S129A and wild-type GRK5 or GRK5–K215R. pS129–αS was seen only in the cells expressing both wild-type GRK5 and wild-type αS. The cells expressing either αS–S129A and GRK5 or αS and GRK5–K215R did not produce pS129–αS. C, Physical association of GRK5 with αS in cultured cells and human brain tissue. A cross-linker, DSP, was used in all experiments. Left and middle, HEK293 cells were transfected with αS and GRK5–FLAG cDNAs, and cell lysate was immunoprecipitated (IP) with control IgG, Syn-1, and anti-FLAG antibody (left). HEK293 cells were also transfected with SOD1–FLAG and GRK5–FLAG, and the cell lysates were immunoprecipitated with anti-SOD1 or anti-GRK5 (H-64) antibodies (middle). Immunoprecipitates were analyzed by immunoblotting with anti-FLAG antibody (top) or with anti-SOD1 antibody (bottom). Binding of GRK5 to αS was detected (left), whereas nonspecific binding of GRK5 to endogenous/overexpressed SOD1 was not (left and middle). Right, Tissue extracts from human temporal cortex were immunoprecipitated with anti-FLAG (negative control), Syn-1, or anti-GRK5 (H-64), followed by immunoblotting with H-64. Binding of GRK5 and αS was detected in the extracts.
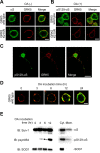
Figure 3.
αS colocalizes with GRK5 at the plasma membrane of HEK293 cells. A, B, HEK293–αS cells were transiently transfected with either wild-type GRK5–FLAG (top) or GRK5–K215R-FLAG (bottom) and incubated in the presence (B) or absence (A) of OA. Fixed cells were processed for direct immunofluorescence with polyclonal anti-GRK5 antibody (H-64) (A and B, red channel) and either monoclonal anti-αS antibody (LB509; A, green channel) or monoclonal anti-pS129–αS antibody (psyn#64; B, green channel). In HEK293–αS cells expressing GRK5, αS and GRK5 colocalized in the area of the plasma membrane (A, top), and pS129–αS was distributed in the areas of the perikarya and plasma membrane (B, top). In the cells expressing mutant GRK5–K215R, there was no fluorescence of pS129-αS, although the distribution of GRK5–K215R was similar to that of wild-type GRK5 (B, bottom). Scale bars, 10 μm. C, Phosphorylation of αS by GRK5 in primary neurons from the cerebral cortex of fetal mice. The primary cortical neurons were cotransfected with wild-type αS and GRK5 cDNAs, treated with 10 n
m
OA for 12 h, and immunostained with anti-pS129–αS (psyn#64; left) and anti-GRK5 (H-64; middle) antibodies. A merged image is shown at right. Scale bar, 20 μm. D, Translocation of pS129–αS catalyzed by GRK5. HEK293–αS cells were transiently transfected with wild-type GRK5–FLAG cDNA, and the cells were incubated with 20 n
m
OA for various time periods. Fixed cells were immunostained with anti-GRK5 (H-64; red) and anti-pS129–αS (psyn#64; green) antibodies. After 3 h of incubation, pS129–αS was detected in the area of the plasma membrane. With time, pS129–αS was gradually translocated from the plasma membrane to the perikaryal area. Scale bar, 10 μm. E, Immunoblotting (IB) of the cytosol and membrane fractions of HEK293–αS cells transiently transfected with wild-type GRK5 cDNA. After incubation of the cells with 20 n
m
OA for various time periods, the cells were disrupted, and the homogenate was sequentially centrifuged at 800 × g for 10 min and at 100,000 × g for 30 min. The fractions were tested for the presence of pS129–αS by immunoblotting. In the presence of OA, the amount of pS129–αS in the cytosol fraction gradually increased with time, although the expression levels of the total αS and SOD1 were constant (left). In the same preparation of cell homogenate at 12 h of OA incubation, the amount of pS129–αS in the cytosol fraction (Cyt.) was much larger than that in the membrane fraction (Mem.; right). Abundant SOD1 was detectable in the cytosol fraction.
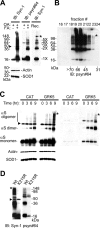
Figure 4.
GRK5 promotes α-linolenic acid-induced oligomerization of αS. A, To test the effect of fatty acid (FA) on αS oligomerization, HEK293–αS cells expressing GRK5–FLAG were preincubated with 20 n
m
OA for 8 h and then conditioned with or without α-linolenic acid for 16 h along with OA. High-speed soluble cytosol fractions (30 μg of protein) from cell lysates were heat treated (65°C for 16 h) to remove lipids before gel loading and were immunoblotted (IB) with Syn-1 (top left) or psyn#64 (top middle). For loading control, the same amounts of non-heat-treated samples were immunoblotted with anti-actin and anti-SOD1 antibodies (bottom left). As the molecular weight standards, αS oligomers formed in vitro by the treatment of recombinant αS (Rec.) with α-linolenic acid (see Materials and Methods) are shown at right. B, High-speed soluble cytosol from cells expressing αS and GRK5 was loaded onto a Superdex 75 column and eluted with ammonium acetate buffer. A large amount of pS129–αS oligomers was eluted into the fractions of high molecular weights (45–70 kDa), indicating that the formation of pS129–αS oligomers occurred under nondenatured conditions. Immunoblotting was made with psyn#64 after heat treatment. C, HEK293–αS cells expressing either GRK5–FLAG or CAT were incubated with 20 n
m
OA for 16 h and conditioned with α-linolenic acid for 0, 3, 6, and 9 h along with OA. High-speed soluble cytosol fractions (30 μg of protein) from cell lysates were heat treated (65°C for 16 h) to remove lipids before gel loading and immunoblotted with Syn-1 (left) or psyn#64 (right). Although the levels of αS monomers and oligomers dominantly containing nonphosphorylated species increased within 3 h in CAT-expressing cells, αS containing phosphorylated species yielded more oligomers in GRK5-expressing cells at 9 h. The smear pattern in the background and the gel-excluded immunoreactive material, which seemed to represent aggregated αS species, were also seen at 9 h after incubation of α-linolenic acid in the presence of GRK5. For loading control, the same amounts of non-heat-treated samples were immunoblotted with anti-actin and anti-SOD1 antibodies (bottom left). D, Expression of the enzymatic-inactive mutant GRK5 (K215R) in HEK293–αS cells, instead of wild-type (Wt) GRK5, failed to promote the oligomerization of αS. The cells transfected with either wild-type GRK5 or GRK5 K215R cDNA were preincubated with 20 n
m
OA for 16 h and incubated for 8 h with α-linolenic acid along with OA. The high-speed soluble cytosol fractions of the cells were analyzed by immunoblotting. Asterisks (A–D) indicate the interface of resolving/stacking gel, and the arrowheads (A, C, D) point to a nonspecific band (∼47 kDa) by Syn-1 antibody (Perrin et al., 2003).
Figure 5.
Haplotype analysis of the GRK5 gene. A, Names and positions of genotyped SNPs in the GRK5 gene (m19, rs10886424; m20, rs11198856; m21, rs1473799; m22, rs871196; m22.1, rs2420616; m23, rs7069375; m24, rs4752293; m25, rs884970; m26, rs291979; m27, rs2275036; m28, rs11198907; m29, rs3740564; m30, rs933048; m31, rs11198922; m32, rs12718341; m33, rs1999628). B–D, The results of HTR analysis in the m22-m23-m24 (B), m22.1-m23-m24 (C), and m22-m22.1-m23-m24 (D) blocks are shown. Haplotypes showing significant empirical p value (10,000 permutations) are shown in bold. ORs were calculated by mean response values (case, 1; control, 0). Overall haplotype empirical p values (10,000 permutations) were 0.0152, 0.0053, and 0.0127 for the blocks m22-m23-m24, m22.1-m22-m24, and m22-m22.1-m23-m24, respectively.
Figure 6.
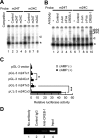
Allelic difference in SNP m24 (T/C). A, EMSA. Double-stranded oligonucleotide with the sequence of SNP m24 T allele (m24T, 5′cagaagactcTgtcatcaggc; lanes 1–4) or m24 C allele (m24C, 5′cagaagactcCgtcatcaggc; lanes 5–8) was 32P labeled and incubated with nuclear extract from SH-SY5Y cells with (lanes 2–4, 6–8) or without (lanes 1, 5) 200-fold excess of nonlabeled competitors. Shift bands (arrowhead) were seen in lanes 1 and 5, and the intensity was higher in m24C (lane 5) than in m24T (lane 1), indicating that m24C had a higher affinity to nuclear protein(s) than m24T. The higher affinity of m24C than m24T was supported by a competition assay, which showed that shift bands were more efficiently inhibited by nonlabeled m24C (lanes 4, 8) than by nonlabeled m24T (lanes 3, 7). An unrelated control sequence did not inhibit shift bands (lanes 2, 6). The asterisk indicates nonspecific shifted bands. B, Supershift assay. 32P-labeled m24T (lanes 1–5) or m24C (lanes 6–10) was incubated with nuclear extract from SH-SY5Y cells with (lanes 2–5, 7–10) or without (lanes 1, 6) antibodies. Anti-CREB-1 antibody diminished the intensity of shift bands (arrowhead; lanes 5, 10) and produced two supershifted bands (double arrowhead; lanes 5, 10). Anti-c-Jun antibody also produced a supershifted band, albeit weaker than CREB-1 (triple arrowhead; lanes 3, 8). The intensity of supershifted bands was higher in m24C (lanes 8, 10) than in m24T (lanes 3, 5). A control anti-STAT-1 antibody did not affect the intensity of shift bands, nor did it produce supershifted bands (lanes 2, 7). C, Luciferase assay. A DNA fragment corresponding to m24T or m24C was inserted into pGL-3 promoter vector to produce reporter with single m24T or m24C and three-tandem m24T or m24C (supplemental Fig. 2, available at
as supplemental material). The reporters were transfected into SH-SY5Y cells, and relative luciferase activity (RLA) was assayed. No difference in RLA was seen among the five constructs in the absence of cAMP. In the presence of cAMP, m24C showed a 1.5-fold increase in RLA compared with m24T (*p < 0.05), and m24C × 3 showed a 9.7-fold increase compared with m24T × 3 (**p < 0.0001). D, ChIP assay. DNA–protein complexes were immunoprecipitated with no antibody (lane 1), unrelated antibody (lane 2), or anti-CREB-1 antibody (lane 3). Lane 3 shows the binding of CREB-1 to the m24 region of the GRK5 gene within intact SH-SY5Y cells. Lane 4 shows input DNA. Experiments were done in duplicate.
Figure 7.
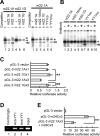
Allelic difference in SNP m22.1 (A/G). A, Left, EMSA. Double-stranded oligonucleotide with the sequence of SNP m22.1 A allele (m22.1A, 5′taaactcagatAtggcttcaggg; lanes 1–3) or m22.1 G allele (m22.1G, 5′taaactcagatGtggcttcaggg; lanes 4–6) was 32P labeled and incubated with nuclear extract from SH-SY5Y cells with (lanes 2, 3, 5, 6) or without (lanes 1, 4) 400-fold excess of nonlabeled competitors. Shift bands (single and double arrowheads) were seen in m22.1A (lanes 1–3) but not in m22.1G (lanes 4, 5). The intensity of shift bands was diminished by addition of competitor m22.1A (lane 2) but not by m22.1G (lane 3), suggesting the binding of nuclear protein(s) to m22.1A. The asterisks indicate nonspecific shifted bands. Right, Competition assays using oligonucleotide with YY1-binding motif. 32P-labeled m22.1A oligonucleotide (5′taaactcagatAtggcttcaggg), which contained a core consensus sequence of YY1-binding site, was incubated with nuclear extract from SH-SY5Y cells with (lanes 2–6) or without (lane 1) 400-fold excess of nonlabeled competitors. The shift band (double arrowhead) was markedly abolished by addition of nonlabeled representative YY1-binding consensus sequence (YY1co; 5′cagccgccaagatggccggggag; lane 5) but not by nonlabeled mutated YY1-binding consensus sequence (YY1mu; 5′cagccgccaagataatcgcggag; lane 6) or by nonlabeled mutated m22.1A (m22.1Amu, 5′taaactcagatAcaccttcaggg; lane 3). B, Supershift assay. m22.1A (lanes 1, 2), m22.1Amu (lanes 3, 4), m22.1G (lanes 5, 6), YY1co (lanes 7, 8), or YY1mu (lanes 9, 10) oligonucleotide labeled with 32P was incubated with nuclear extract from SH-SY5Y cells with (lanes 2, 4, 6, 8, 10) or without (lanes 1, 3, 5, 7, 9) antibody against YY1. The shift band (double arrowhead) was produced by m22.1A (lane 1) or YY1co (lane 7) oligonucleotide labeled with 32P; this band was markedly abolished by the antibody (lanes 2, 8), suggesting the binding of YY1 to m22.1A as well as YY1co. C, Luciferase assay. Single- or three-concatenated oligonucleotide with m22.1A or m22.1G was inserted into pGL-3 promoter vector (supplemental Fig. 2, available at
as supplemental material), and relative luciferase activity (RLA) was assayed. m22.1A × 3 showed a 42% decrease in RLA compared with m22.1G × 3 (**p < 0.0001), indicating a repressed transcriptional activity by YY1. D, ChIP assay. DNA–protein complexes were immunoprecipitated with either unrelated antibody (lane 1) or anti-YY1 antibody (lanes 2, 3). Lanes 2 and 3 show the binding of YY1 to the m22.1 region of the GRK5 gene within intact SH-SY5Y cells. Lane 4 shows input DNA. Experiments were done in duplicate. E, A combination effect of two SNPs (supplemental Fig. 2, available at
as supplemental material) on the expression of the reporter gene, showing that the increased activity of luciferase by m24C × 3 was suppressed by the addition of m22.1A × 3 (**p < 0.0001). Experiments were performed in triplicate.
Similar articles
- Identification of G-protein coupled receptor kinase 2 in paired helical filaments and neurofibrillary tangles.
Takahashi M, Uchikado H, Caprotti D, Weidenheim KM, Dickson DW, Ksiezak-Reding H, Pasinetti GM. Takahashi M, et al. J Neuropathol Exp Neurol. 2006 Dec;65(12):1157-69. doi: 10.1097/01.jnen.0000248542.82681.12. J Neuropathol Exp Neurol. 2006. PMID: 17146290 - G protein-coupled receptor kinase 5, overexpressed in the alpha-synuclein up-regulation model of Parkinson's disease, regulates bcl-2 expression.
Liu P, Wang X, Gao N, Zhu H, Dai X, Xu Y, Ma C, Huang L, Liu Y, Qin C. Liu P, et al. Brain Res. 2010 Jan 11;1307:134-41. doi: 10.1016/j.brainres.2009.10.036. Epub 2009 Oct 21. Brain Res. 2010. PMID: 19852948 - In vivo modulation of polo-like kinases supports a key role for PLK2 in Ser129 α-synuclein phosphorylation in mouse brain.
Bergeron M, Motter R, Tanaka P, Fauss D, Babcock M, Chiou SS, Nelson S, San Pablo F, Anderson JP. Bergeron M, et al. Neuroscience. 2014 Jan 3;256:72-82. doi: 10.1016/j.neuroscience.2013.09.061. Epub 2013 Oct 12. Neuroscience. 2014. PMID: 24128992 - α-Synuclein phosphorylation as a therapeutic target in Parkinson's disease.
Braithwaite SP, Stock JB, Mouradian MM. Braithwaite SP, et al. Rev Neurosci. 2012 Mar 21;23(2):191-8. doi: 10.1515/revneuro-2011-0067. Rev Neurosci. 2012. PMID: 22499677 Review. - Parkinson's disease and alpha synuclein: is Parkinson's disease a prion-like disorder?
Olanow CW, Brundin P. Olanow CW, et al. Mov Disord. 2013 Jan;28(1):31-40. doi: 10.1002/mds.25373. Mov Disord. 2013. PMID: 23390095 Review.
Cited by
- The Interplay between Alpha-Synuclein Clearance and Spreading.
Lopes da Fonseca T, Villar-Piqué A, Outeiro TF. Lopes da Fonseca T, et al. Biomolecules. 2015 Apr 14;5(2):435-71. doi: 10.3390/biom5020435. Biomolecules. 2015. PMID: 25874605 Free PMC article. Review. - Uncovering conserved networks and global conformational changes in G protein-coupled receptor kinases.
Seo MJ, Yu W. Seo MJ, et al. Comput Struct Biotechnol J. 2024 Sep 28;23:3445-3453. doi: 10.1016/j.csbj.2024.09.014. eCollection 2024 Dec. Comput Struct Biotechnol J. 2024. PMID: 39403406 Free PMC article. - Yin Yang 1 expression in the adult rodent brain.
Rylski M, Amborska R, Zybura K, Konopacki FA, Wilczynski GM, Kaczmarek L. Rylski M, et al. Neurochem Res. 2008 Dec;33(12):2556-64. doi: 10.1007/s11064-008-9757-y. Epub 2008 Jun 27. Neurochem Res. 2008. PMID: 18584324 - G protein-coupled receptor kinases: more than just kinases and not only for GPCRs.
Gurevich EV, Tesmer JJ, Mushegian A, Gurevich VV. Gurevich EV, et al. Pharmacol Ther. 2012 Jan;133(1):40-69. doi: 10.1016/j.pharmthera.2011.08.001. Epub 2011 Aug 26. Pharmacol Ther. 2012. PMID: 21903131 Free PMC article. Review. - Phosphorylation at Ser-129 but not the phosphomimics S129E/D inhibits the fibrillation of alpha-synuclein.
Paleologou KE, Schmid AW, Rospigliosi CC, Kim HY, Lamberto GR, Fredenburg RA, Lansbury PT Jr, Fernandez CO, Eliezer D, Zweckstetter M, Lashuel HA. Paleologou KE, et al. J Biol Chem. 2008 Jun 13;283(24):16895-905. doi: 10.1074/jbc.M800747200. Epub 2008 Mar 14. J Biol Chem. 2008. PMID: 18343814 Free PMC article.
References
- Arawaka S, Hasegawa H, Tandon A, Janus C, Chen F, Yu G, Kikuchi K, Koyama S, Kato T, Fraser PE, St George-Hyslop P. The levels of mature glycosylated nicastrin are regulated and correlate with gamma-secretase processing of amyloid beta-precursor protein. J Neurochem. 2002;83:1065–1071. - PubMed
- Bucciantini M, Giannoni E, Chiti F, Baroni F, Formigli L, Zurdo J, Taddei N, Ramponi G, Dobson CM, Stefani M. Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature. 2002;416:507–511. - PubMed
- Chen L, Feany MB. Alpha-synuclein phosphorylation controls neurotoxicity and inclusion formation in a Drosophila model of Parkinson disease. Nat Neurosci. 2005;8:657–663. - PubMed
- Chung KK, Dawson VL, Dawson TM. New insights into Parkinson's disease. J Neurol. 2003;250([Suppl 3]):III15–III24. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials