Mode of functional connectivity in amygdala pathways dissociates level of awareness for signals of fear - PubMed (original) (raw)
Randomized Controlled Trial
Mode of functional connectivity in amygdala pathways dissociates level of awareness for signals of fear
Leanne M Williams et al. J Neurosci. 2006.
Abstract
Many of the same regions of the human brain are activated during conscious attention to signals of fear and in the absence of awareness for these signals. The neural mechanisms that dissociate level of awareness from activation in these regions remain unknown. Using functional magnetic resonance imaging with connectivity analysis in healthy human subjects, we demonstrate that level of awareness for signals of fear depends on mode of functional connectivity in amygdala pathways rather than discrete patterns of activation in these pathways. Awareness for fear relied on negative connectivity within both cortical and subcortical pathways to the amygdala, suggesting that reentrant feedback may be necessary to afford such awareness. In contrast, responses to fear in the absence of awareness were supported by positive connections in a direct subcortical pathway to the amygdala, consistent with the view that excitatory feedforward connections along this pathway may be sufficient for automatic responses to "unseen" fear.
Figures
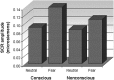
Figure 1.
Skin conductance responses elicited by fear relative to neutral under conscious and nonconscious perception conditions. The amplitude of SCRs is shown in microsiemens. Fear elicited enhanced SCRs relative to neutral under both awareness conditions.
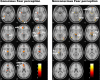
Figure 2.
Statistical parameter maps for the regions of interest activated in response to conscious fear (left) (vs neutral) and nonconscious fear (right) (vs neutral). For conscious fear, significant (p < 0.05, small-volume corrected) activations were observed in the amygdala, thalamus (including LGN), anterior cingulate (dorsal and ventral portions), and visual cortices, both striate and extrastriate (including fusiform and inferior occipital gyri). Activations were also observed in the superior colliculus and hypothalamus regions of the brainstem. For nonconscious fear, significant (p < 0.05, small-volume corrected) activity was observed in the bilateral amygdala and brainstem regions consistent with the superior colliculus and hypothalamus. Activity was also observed in the thalamic pulvinar (rather than LGN), in the ventral (but not dorsal) anterior cingulate, and in the extrastriate (right inferior occipital gyrus) but not striate visual cortex. Coordinates for these regions of significant activation are presented in Table 1. A, Amygdala; V1, striate visual cortex; SC, superior colliculus of the brainstem; Pulv, pulvinar of the thalamus; Th, thalamus; Hy, hypothalamus; dMPFC, dorsal MPFC; vMPFC, ventral MPFC.
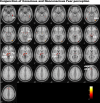
Figure 3.
Statistical parameter maps for the conjunction of activation in regions of interest to both conscious and nonconscious fear (vs neutral). In conjunction, these conditions showed significant (p < 0.05, small-volume corrected) activations in the amygdala, thalamus (encompassing regions consistent with LGN and pulvinar), brainstem, and extrastriate GOi and Gf regions. Small regions of common, trend-level activation were also observed in a dorsal portion of the MPFC and ACC. There were no corresponding clusters of common activity in the ventral prefrontal or striate visual cortices. A, Amygdala; dMPFC, dorsal MPFC; Hy, hypothalamus; Pulv, pulvinar of the thalamus; SC, superior colliculus of the brainstem; Th, thalamus.
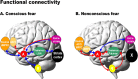
Figure 4.
Psychophysiological interaction analyses showing the functional connectivity between the amygdala and the regions of interest during conscious fear (A) and nonconscious fear (B) relative to neutral. Red arrows represent positive functional connectivity between the amygdala and regions of interest, and blue arrows represent negative connectivity. Conscious fear (A) was distinguished by negative functional connectivity in a cortical (striate–thalamic LGN) amygdala pathway, with links to both left and right amygdala. Negative connectivity was also observed between the bilateral amygdala and other cortical regions (extrastriate and dorsal medial prefrontal), as well as the subcortical brainstem region. Conscious fear elicited positive functional connectivity between the bilateral amygdala and dorsal ACC. The right amygdala in particular also covaried positively with the ventral ACC, extrastriate, and periaqueductal gray regions of the brainstem (data not shown). Nonconscious fear (B), in contrast, elicited positive functional connectivity in a subcortical (brainstem–thalamic pulvinar) amygdala pathway, localized to the right amygdala. Positive relationships were also observed between the bilateral amygdala and rostral regions of the medial prefrontal and ACC. For nonconscious fear, the right amygdala showed negative relationships with additional cortical regions: the extrastriate and ventral medial prefrontal cortices (data not shown). A, Amygdala; B, brainstem; dACC, dorsal ACC; dMPFC, dorsal MPFC; vMPFC, ventral MPFC.
Similar articles
- A direct brainstem-amygdala-cortical 'alarm' system for subliminal signals of fear.
Liddell BJ, Brown KJ, Kemp AH, Barton MJ, Das P, Peduto A, Gordon E, Williams LM. Liddell BJ, et al. Neuroimage. 2005 Jan 1;24(1):235-43. doi: 10.1016/j.neuroimage.2004.08.016. Neuroimage. 2005. PMID: 15588615 - Awareness of Emotional Stimuli Determines the Behavioral Consequences of Amygdala Activation and Amygdala-Prefrontal Connectivity.
Lapate RC, Rokers B, Tromp DP, Orfali NS, Oler JA, Doran ST, Adluru N, Alexander AL, Davidson RJ. Lapate RC, et al. Sci Rep. 2016 May 16;6:25826. doi: 10.1038/srep25826. Sci Rep. 2016. PMID: 27181344 Free PMC article. - A subcortical pathway to the right amygdala mediating "unseen" fear.
Morris JS, Ohman A, Dolan RJ. Morris JS, et al. Proc Natl Acad Sci U S A. 1999 Feb 16;96(4):1680-5. doi: 10.1073/pnas.96.4.1680. Proc Natl Acad Sci U S A. 1999. PMID: 9990084 Free PMC article. - The role of the amygdala in human fear: automatic detection of threat.
Ohman A. Ohman A. Psychoneuroendocrinology. 2005 Nov;30(10):953-8. doi: 10.1016/j.psyneuen.2005.03.019. Psychoneuroendocrinology. 2005. PMID: 15963650 Review. - On the unconscious subcortical origin of human fear.
Ohman A, Carlsson K, Lundqvist D, Ingvar M. Ohman A, et al. Physiol Behav. 2007 Sep 10;92(1-2):180-5. doi: 10.1016/j.physbeh.2007.05.057. Epub 2007 May 25. Physiol Behav. 2007. PMID: 17599366 Review.
Cited by
- Task modulated brain connectivity of the amygdala: a meta-analysis of psychophysiological interactions.
Di X, Huang J, Biswal BB. Di X, et al. Brain Struct Funct. 2017 Jan;222(1):619-634. doi: 10.1007/s00429-016-1239-4. Epub 2016 Jun 3. Brain Struct Funct. 2017. PMID: 27259584 Free PMC article. - Prestimulus amygdala spectral activity is associated with visual face awareness.
Guex R, Ros T, Mégevand P, Spinelli L, Seeck M, Vuilleumier P, Domínguez-Borràs J. Guex R, et al. Cereb Cortex. 2023 Feb 7;33(4):1044-1057. doi: 10.1093/cercor/bhac119. Cereb Cortex. 2023. PMID: 35353177 Free PMC article. - Neural mechanisms of expectancy-based placebo effects in antidepressant clinical trials.
Zilcha-Mano S, Wang Z, Peterson BS, Wall MM, Chen Y, Wager TD, Brown PJ, Roose SP, Rutherford BR. Zilcha-Mano S, et al. J Psychiatr Res. 2019 Sep;116:19-25. doi: 10.1016/j.jpsychires.2019.05.023. Epub 2019 May 26. J Psychiatr Res. 2019. PMID: 31176108 Free PMC article. Clinical Trial. - Ketamine's acute effects on negative brain states are mediated through distinct altered states of consciousness in humans.
Hack LM, Zhang X, Heifets BD, Suppes T, van Roessel PJ, Yesavage JA, Gray NJ, Hilton R, Bertrand C, Rodriguez CI, Deisseroth K, Knutson B, Williams LM. Hack LM, et al. Nat Commun. 2023 Oct 19;14(1):6631. doi: 10.1038/s41467-023-42141-5. Nat Commun. 2023. PMID: 37857620 Free PMC article. Clinical Trial. - Mapping neural activity patterns to contextualized fearful facial expressions onto callous-unemotional (CU) traits: intersubject representational similarity analysis reveals less variation among high-CU adolescents.
Rhoads SA, Cardinale EM, O'Connell K, Palmer AL, VanMeter JW, Marsh AA. Rhoads SA, et al. Personal Neurosci. 2020 Nov 10;3:e12. doi: 10.1017/pen.2020.13. eCollection 2020. Personal Neurosci. 2020. PMID: 33283146 Free PMC article.
References
- Attwell D, Iadecola C. The neural basis of functional brain imaging signals. Trends Neurosci. 2002;25:621–625. - PubMed
- Baddeley A, Emslie H, Nimmo-Smith I. The Spot-the-Word test: a robust estimate of verbal intelligence based on lexical decision. Br J Clin Psychol. 1993;32:55–65. - PubMed
- Bush P, Sejnowski T. Inhibition synchronizes sparsely connected cortical neurons within and between columns in realistic network models. J Comput Neurosci. 1996;3:91–110. - PubMed
- Catani M, Jones DK, Donato R, Ffytche DH. Occipito-temporal connections in the human brain. Brain. 2003;126:2093–2107. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources