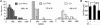The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons? - PubMed (original) (raw)
The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons?
Elyssa B Margolis et al. J Physiol. 2006.
Abstract
The ventral tegmental area (VTA) and in particular VTA dopamine (DA) neurons are postulated to play a central role in reward, motivation and drug addiction. However, most evidence implicating VTA DA neurons in these functions is based on indirect electrophysiological characterization, rather than cytochemical identification. These physiological criteria were first established in the substantia nigra pars compacta (SNc), but their validity in the VTA is uncertain. In the current study we found that while 88 +/- 2% of SNc neurons labelled by the neuronal marker NeuN were co-labelled for the catecholamine enzyme tyrosine hydroxylase (TH), a much smaller percentage (55 +/- 2%) of VTA neurons co-expressed TH. In addition, using in vitro whole-cell recordings we found that widely accepted physiological criteria for VTA DA neurons, including the hyperpolarization-activated inwardly rectifying non-specific cation current (I(h)), spike duration, and inhibition by DA D2 receptor agonists, do not reliably predict the DA content of VTA neurons. We could not distinguish DA neurons from other VTA neurons by size, shape, input resistance, I(h) size, or spontaneous firing rate. Although the absence of an I(h) reliably predicted that a VTA neuron was non-dopaminergic, and I(h)(-) neurons differ from I(h)(+) neurons in firing rate, interspike interval (ISI) standard deviation, and ISI skew, no physiological property examined here is both sensitive and selective for DA neurons in the VTA. We conclude that reliable physiological criteria for VTA DA neuron identification have yet to be determined, and that the criteria currently being used are unreliable.
Figures
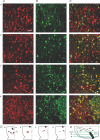
Figure 1. Subregions of the VTA vary greatly in their neural density and DA neuron content
A and B, NeuN (red) and TH (green) immunocytochemistry in various locations in the VTA (scale bar: 50 μm). C, overlays of NeuN and TH result in varied percentages of co-labelling (yellow) depending on VTA subregion. D, locations in the horizontal plane (red, not to scale). a, b, c and d correspond to those panels of the confocal scans in A, B and C. In each panel of D, right is lateral, left is midline, and top is rostral. Scans are from ventral (a), middle (b), and dorsal (c,d) sections. E, dorso-ventral location of the horizontal slices containing scans a–d. Medial is to the right, dorsal is up. MT, medial terminal nucleus of the accessory optic tract; mt, mammillary tract; IPF, interpeduncular fossa; SNc, substantia nigra pars compacta; SNr, substantia nigra pars reticulata.
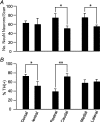
Figure 2. Dorsal and caudal VTA contain the greatest percentage of TH(+) neurons
A, neurons are significantly more densely packed in the rostal than caudal (n = 17 and n = 16 scans, respectively) and medial compared to lateral (n = 18 and n = 26 scans, respectively) VTA. There was no difference between dorsal and ventral VTA neuron density (n = 19 and n = 12 scans, respectively). B, the percentage of TH(+) neurons is significantly greater in the dorsal and caudal VTA. ANOVA: *P < 0.05; **P < 0.0005.
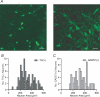
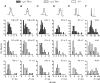
Figure 3. Neuron size does not sort by neurotransmitter content in the VTA
A, sample confocal images from GAD67 immunocytochemistry in horizontal brain slices containing the VTA show the various shapes and sizes of GABAergic neurons in the VTA (scale bar: 30 μm). B, cross-sectional areas of TH(+) and GAD67(+) cell bodies in the VTA have overlapping size distributions.
Figure 4. Distribution of electrophysiological recordings in the VTA
Whole-cell recordings were made in 150 μm horizontal brain sections containing the VTA. In each panel, right is midline, left is lateral, and top is rostral. Neurons were categorized here by their TH content evaluated by cytochemistry and the presence or absence of an _I_h. The boundary between rostral and caudal VTA was taken as the middle of the MT, marked by the horizontal grey dashed line in each slice, which corresponded to −5.3, –5.5 and −5.5 mm from Bregma in the ventral, middle and dorsal VTA slices, respectively (Paxinos & Watson, 1998).
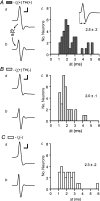
Figure 5. _I_h is similar between dopaminergic and non-dopaminergic neurons
A, example _I_h(+) neuron filled with biocytin (red; scale bar: 40 μm) during recording and immunocytochemically identified to be TH(+) (green, yellow; scale bars: 200 pA and 50 ms). B, example _I_h(+) neuron that was TH(−) (scale bars: 200 pA and 50 ms). C, example _I_h(−) neuron that was TH(−) (scale bars: 20 pA and 50 ms). D, the current induced by stepping the cells in voltage clamp to a variety of membrane potentials was not different between TH(+) and TH(−) _I_h(+) neurons. Inset, the _I_h was measured as the difference between the initial response to the step and the end of each 200 ms pulse. E, the magnitude of the _I_h due to a step from −60 to −120 mV was not different between TH(+) and TH(−) _I_h(+) neurons (n = 46 and n = 41, respectively). _I_h(−) neurons were excluded from this analysis.
Figure 6. The input resistance of _I_h(−) neurons is greater than that of _I_h(+) neurons
Input resistance was measured in current clamp mode with hyperpolarizing pulses. A, the input resistance of _I_h(−) neurons is significantly greater than either TH(+) or TH(−) _I_h(+) neurons, whose input resistances are similar (_I_h(+) TH(+) _versus I_h(−), P < 0.0001; _I_h(+) TH(−) _versus I_h(−), P < 0.000005; _I_h(+) TH(+) _versus I_h(+) TH(−), P = 0.58). B, the cross-sectional area of _I_h(−) TH(−) neurons, as measured during recordings, was significantly smaller than those of either TH(+) or TH(−) _I_h(+) neurons (n = 16, 28 and 28, respectively). *P < 0.001; **P < 0.0005.
Figure 7. Action potential duration does not differ among TH(+) and TH(−) VTA neurons
There were no differences among _I_h(+) TH(+), _I_h(+) TH(−), and _I_h(−) neurons in action potential time to peak (A), duration at half-height (B), or width at base (C) (n = 35, 33 and 26, respectively). In neurons exhibiting a prominent Ca2+-gated fast K+ current, there was no difference among these types of cells in time to the fast K+ minimum (D) or time to the _I_K(AHP) (E) (n = 23, 22 and 21 for _I_h(+) TH(+), _I_h(+) TH(−), and _I_h(−) neurons, respectively). F, the time to the peak (minimum) of the afterhyperpolarization was significantly longer in _I_h(−) neurons than in either TH(+) or TH(−) _I_h(+) neurons (_I_h(+) TH(+) _versus I_h(−) and _I_h(+) TH(−) _versus I_h(−), P < 0.05).
Figure 8. Differentiated whole-cell action potentials, a correlate of extracellular action potentials, are similar among VTA neurons
From _I_h(+) TH(+) (A), _I_h(+) TH(−) (B) and _I_h(−) (C) neurons: in each case example action potentials recorded in whole-cell configuration (I = 0; a) are shown (scale bars: 20 mV and 2 ms). The derivatives of these action potentials correspond to extracellularly recorded action potentials (b). c, the duration of these derived action potentials from initiation to the trough minimum is similar in all 3 types of neurons (n = 35, 32 and 26 for _I_h(+) TH(+), _I_h(+) TH(−), and _I_h(−) neurons, respectively).
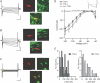
Figure 9. Firing pattern properties are different between _I_h(+) dopaminergic and non-dopaminergic neurons
A, stably spontaneously active _I_h(−) neurons (n = 10) have a higher firing rate than either group of _I_h(+) neurons (TH(+) n = 25; TH(−) n = 18; _I_h(+) TH(+) _versus I_h(−) and _I_h(+) TH(−) _versus I_h(−), P < 0.0005). B, in these neurons, the mean standard deviation of interspike intervals (ISIs) from a train of 601 consecutive action potentials is not different among cell types, although most _I_h(−) neurons exhibit very small deviations (ANOVA: P = 0.13). C, the skew of the same sample of ISIs is significantly higher in _I_h(−) neurons than in _I_h(+) neurons (_I_h(+) TH(+) _versus I_h(−), P < 0.001; and _I_h(+) TH(−) _versus I_h(−), P < 0.005). D, there is an inverse relationship between firing rate and ISI standard deviation shared by the three cell types (note the exponential scales of axis and ordinate). E, there is a relationship between ISI skew and firing rate in spontaneously active VTA neurons.
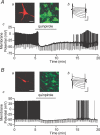
Figure 10. Both dopaminergic and non-dopaminergic VTA neurons are inhibited by dopamine Example neurons with biocytin fills (a; red; scale bar: 40 μm) and TH staining (a; green), _I_h (b; scale bars: 200 pA and 50 ms), and response to bath application of the D2 agonist quinpirole (c; 1 μm)
A, an example TH(+) neuron is inhibited and hyperpolarized by quinpirole application. B, a sample TH(−) neuron is also inhibited and hyperpolarized by quinpirole.
Similar articles
- Physiological properties of zebra finch ventral tegmental area and substantia nigra pars compacta neurons.
Gale SD, Perkel DJ. Gale SD, et al. J Neurophysiol. 2006 Nov;96(5):2295-306. doi: 10.1152/jn.01040.2005. Epub 2006 Jul 26. J Neurophysiol. 2006. PMID: 16870835 - Differential modulation by nicotine of substantia nigra versus ventral tegmental area dopamine neurons.
Keath JR, Iacoviello MP, Barrett LE, Mansvelder HD, McGehee DS. Keath JR, et al. J Neurophysiol. 2007 Dec;98(6):3388-96. doi: 10.1152/jn.00760.2007. Epub 2007 Oct 17. J Neurophysiol. 2007. PMID: 17942622 - Intracerebroventricular administration of NMDA-R1 antisense oligodeoxynucleotide significantly alters the activity of ventral tegmental area dopamine neurons: an electrophysiological study.
Tajiri K, Emori K, Murata M, Tanaka K, Suzuki M, Uehara T, Sumiyoshi T, Ashby CR Jr, Kurachi M. Tajiri K, et al. Synapse. 2001 Jun 15;40(4):275-81. doi: 10.1002/syn.1050. Synapse. 2001. PMID: 11309843 - Environment- and activity-dependent dopamine neurotransmitter plasticity in the adult substantia nigra.
Aumann TD. Aumann TD. J Chem Neuroanat. 2016 Apr;73:21-32. doi: 10.1016/j.jchemneu.2015.12.009. Epub 2015 Dec 21. J Chem Neuroanat. 2016. PMID: 26718607 Review. - Reward and aversion in a heterogeneous midbrain dopamine system.
Lammel S, Lim BK, Malenka RC. Lammel S, et al. Neuropharmacology. 2014 Jan;76 Pt B(0 0):351-9. doi: 10.1016/j.neuropharm.2013.03.019. Epub 2013 Apr 8. Neuropharmacology. 2014. PMID: 23578393 Free PMC article. Review.
Cited by
- Memantine block depends on agonist presentation at the NMDA receptor in substantia nigra pars compacta dopamine neurones.
Wild AR, Akyol E, Brothwell SL, Kimkool P, Skepper JN, Gibb AJ, Jones S. Wild AR, et al. Neuropharmacology. 2013 Oct;73:138-46. doi: 10.1016/j.neuropharm.2013.05.013. Epub 2013 May 28. Neuropharmacology. 2013. PMID: 23727219 Free PMC article. - Suppression of Gq Function Using Intra-Pipette Delivery of shRNA during Extracellular Recording in the Ventral Tegmental Area.
Nimitvilai S, Arora DS, McElvain MA, Brodie MS. Nimitvilai S, et al. Front Cell Neurosci. 2013 Feb 12;7:7. doi: 10.3389/fncel.2013.00007. eCollection 2013. Front Cell Neurosci. 2013. PMID: 23408114 Free PMC article. - Understanding opioid reward.
Fields HL, Margolis EB. Fields HL, et al. Trends Neurosci. 2015 Apr;38(4):217-25. doi: 10.1016/j.tins.2015.01.002. Epub 2015 Jan 29. Trends Neurosci. 2015. PMID: 25637939 Free PMC article. Review. - Voluntary adolescent drinking enhances excitation by low levels of alcohol in a subset of dopaminergic neurons in the ventral tegmental area.
Avegno EM, Salling MC, Borgkvist A, Mrejeru A, Whitebirch AC, Margolis EB, Sulzer D, Harrison NL. Avegno EM, et al. Neuropharmacology. 2016 Nov;110(Pt A):386-395. doi: 10.1016/j.neuropharm.2016.07.031. Epub 2016 Jul 27. Neuropharmacology. 2016. PMID: 27475082 Free PMC article. - Alcohol effects on synaptic transmission in periaqueductal gray dopamine neurons.
Li C, McCall NM, Lopez AJ, Kash TL. Li C, et al. Alcohol. 2013 Jun;47(4):279-87. doi: 10.1016/j.alcohol.2013.02.002. Epub 2013 Apr 15. Alcohol. 2013. PMID: 23597415 Free PMC article.
References
- Aghajanian GK, Bunney BS. Central dopaminergic neurons – neurophysiological identification and responses to drugs. Life Sci. 1973;13:643–648.
- Cameron DL, Wessendorf MW, Williams JT. A subset of ventral tegmental area neurons is inhibited by dopamine, 5-hydroxytryptamine and opioids. Neuroscience. 1997;77:155–166. - PubMed
- Carr DB, Sesack SR. GABA-containing neurons in the rat ventral tegmental area project to the prefrontal cortex. Synapse. 2000a;38:114–123. - PubMed
- Chudasama Y, Robbins TW. Psychopharmacological approaches to modulating attention in the five-choice serial reaction time task: implications for schizophrenia. Psychopharmacology (Berl) 2004;174:86–98. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous