The selective value of bacterial shape - PubMed (original) (raw)
Review
The selective value of bacterial shape
Kevin D Young. Microbiol Mol Biol Rev. 2006 Sep.
Abstract
Why do bacteria have shape? Is morphology valuable or just a trivial secondary characteristic? Why should bacteria have one shape instead of another? Three broad considerations suggest that bacterial shapes are not accidental but are biologically important: cells adopt uniform morphologies from among a wide variety of possibilities, some cells modify their shape as conditions demand, and morphology can be tracked through evolutionary lineages. All of these imply that shape is a selectable feature that aids survival. The aim of this review is to spell out the physical, environmental, and biological forces that favor different bacterial morphologies and which, therefore, contribute to natural selection. Specifically, cell shape is driven by eight general considerations: nutrient access, cell division and segregation, attachment to surfaces, passive dispersal, active motility, polar differentiation, the need to escape predators, and the advantages of cellular differentiation. Bacteria respond to these forces by performing a type of calculus, integrating over a number of environmental and behavioral factors to produce a size and shape that are optimal for the circumstances in which they live. Just as we are beginning to answer how bacteria create their shapes, it seems reasonable and essential that we expand our efforts to understand why they do so.
Figures
FIG. 1.
Variety of prokaryotic shapes. This collage of different cells, unless otherwise stated, is constructed from descriptions and illustrations given by Starr et al. (313) or by Zinder and Dworkin (380). The cells are drawn to scale. Those in the dashed black circle are drawn relative to the 5-μm line. These same cells are included in smaller form in the dashed blue circle to compare their sizes to those of larger bacteria, which are drawn relative to the 10-μm line. (A) Stella strain IFAM1312 (380); (B) Microcyclus (a genus since renamed Ancylobacter) flavus (367); (C) Bifidobacterium bifidum; (D) Clostridium cocleatum; (E) Aquaspirillum autotrophicum; (F) Pyroditium abyssi (380); (G) Escherichia coli; (H) Bifidobacterium sp.; (I) transverse section of ratoon stunt-associated bacterium; (J) Planctomyces sp. (133); (K) Nocardia opaca; (L) Chain of ratoon stunt-associated bacteria; (M) Caulobacter sp. (380); (N) Spirochaeta halophila; (O) Prosthecobacter fusiformis; (P) Methanogenium cariaci; (Q) Arthrobacter globiformis growth cycle; (R) gram-negative Alphaproteobacteria from marine sponges (240); (S) Ancalomicrobium sp. (380); (T) Nevskia ramosa (133); (U) Rhodomicrobium vanniellii; (V) Streptomyces sp.; (W) Caryophanon latum; (X) Calothrix sp. The yellow-lined background orb represents a slice of the giant bacterium Thiomargarita namibiensis (290), which is represented to scale with the other organisms.
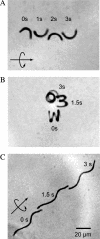
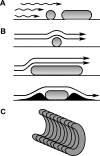
FIG. 2.
Effect of artificially imposed cell shape on motility of Escherichia coli. E. coli filaments were forced into defined shapes by growing the cells in preformed cavities (322). The cells pictured here are genetically and biochemically identical except for differences in helical pitch or curvature. Time-lapse microscopy captured the positions of motile cells as they swam in the indicated directions (straight arrows), moving with a rotary motion (circular arrows) over a few seconds (as indicated by the numbers). (A) Crescent-shaped cell swimming in a straight line. (B) Tightly wound spiral-shaped cell swimming in a counterclockwise circle. (C) Relaxed spiral-shaped cell swimming in a straight line. The cell in panel C was derived from those represented in panel B by incubating the cells outside the original growth chambers for 2 hours. (Reprinted with permission from reference . Copyright 2005 American Chemical Society.)
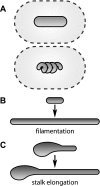
FIG. 3.
Contributions of shape to nutrient acquisition. (A) Approximately equal diffusion spheres may enclose cells of different shapes. (B) Bacteria may respond to nutrient deprivation by filamentation, which increases their total surface area without an appreciable increase in the surface-to-volume ratio. (C) Prosthecate cells may respond to nutrient deprivation by elongating their thin prosthecae, which increases their total surface area while decreasing their surface-to-volume ratio.
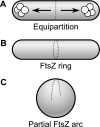
FIG. 4.
How division and segregation help maintain geometrically uniform cell shapes. (A) Geometric uniformity simplifies equipartition of material into daughter cells during cell division. (B) In a wild-type bacillus, the cell division protein FtsZ forms a ring (the Z ring) that encircles the midpoint of the rod-shaped cell and initiates division. (C) A spherical cell derived from the cell in panel B may not be able to form a complete Z ring around the increased circumference of the cell's midpoint.
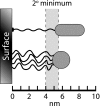
FIG. 5.
Energetics of cell attachment to a surface. Cells stop within a certain distance of a surface because of electrostatic repulsion, where they may be retained within the Gibbs energy “secondary-minimum” zone (shaded area). The specific minima are shown for one species of Corynebacterium approaching a glass surface in a solution with 0.1 M ionic strength (269). The exact location of the secondary energy minimum will vary from 4 to 10 nm, depending on the nature of the surface and the bulk ionic conditions. Cells may initiate direct physical contact with the surface across the energy barrier by using pili (long thin fiber on upper cell) or by secreting polymeric capsular materials (thin fibers on lower cell). (Adapted and redrawn from reference , copyright 1996, with permission from American Urological Association.)
FIG. 6.
Examples of physical considerations affecting attachment of cells to surfaces. (A) Rod-shaped cells can contact a surface with a larger amount of their cell body than can cocci. (B) Shear flow from moving liquid (arrows) may align rod-shaped cells parallel to the flow, so that cell width is the major dimension that is directly affected by the shearing force. If a coccus and a rod present the same face to the oncoming liquid, the rod should be more difficult to remove because it has more connections to the surface. (C) Individual, curved Simonsiella cells are connected to one another to form a distinctive filamentous shape. The organism binds to epithelial cells in the oral cavities of mammals, with the attachment being mediated by the concave face of the cell filament.
FIG. 7.
Cell-to-cell attachments in the formation of daughter cell tetrads and microcolonies. E. coli CS315 cells were inoculated onto a rich agar medium in a microscope chamber and photographed at time intervals while being incubated at 37°C. Incubation time increases from left to right in 10-min increments. This strain lacks penicillin binding proteins 4, 5, and 7 and produces many misshapen cells (50). (A) Cells with the normal rod shape grow and divide, after which the two daughter cells slip and grow along one another's sides to form a typical four-daughter cell tetrad. Subsequent growth of such a microcolony is typified by continued close contact among the cells (M. Larson and K. D. Young, unpublished data). This behavior has been well established by other investigators (59, 296). (B) Cells where at least one daughter is misshapen do not form closely knit daughter tetrads, and the resulting microcolonies often have numerous gaps because of the irregularly shaped cells (M. Larson and K. D. Young, unpublished data).
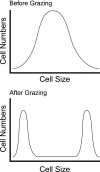
FIG. 8.
Protozoal predation often produces a bimodal pressure on bacterial cell size. (Top) Bacterial cell sizes in the absence of protozoal predation. (Bottom) Bacterial cell sizes after protozoal predation. In the latter case, cells of intermediate size have been removed by grazing, leaving increased numbers of smaller and larger bacteria. (Adapted and redrawn with permission from Nature Reviews Microbiology [246] copyright Macmillan Magazines Ltd.)
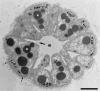
FIG. 9.
Influence of multicellularity on individual cell shapes. Cell shapes in a spherical, multicellular, magnetotactic bacterium (161, 162) are shown. Individual cells are pyramid or cone shaped so that they fit together to form a sphere with a small hollow interior. The shape of each cell is influenced by physical interactions with its neighbors. Bar, 1 μm. (Reprinted from reference with permission from Elsevier.)
Similar articles
- Bacterial morphology: why have different shapes?
Young KD. Young KD. Curr Opin Microbiol. 2007 Dec;10(6):596-600. doi: 10.1016/j.mib.2007.09.009. Epub 2007 Nov 5. Curr Opin Microbiol. 2007. PMID: 17981076 Free PMC article. Review. - Bacterial shape: two-dimensional questions and possibilities.
Young KD. Young KD. Annu Rev Microbiol. 2010;64:223-40. doi: 10.1146/annurev.micro.112408.134102. Annu Rev Microbiol. 2010. PMID: 20825347 Free PMC article. Review. - Thinking about bacterial populations as multicellular organisms.
Shapiro JA. Shapiro JA. Annu Rev Microbiol. 1998;52:81-104. doi: 10.1146/annurev.micro.52.1.81. Annu Rev Microbiol. 1998. PMID: 9891794 Review. - The Molecular Basis of Noncanonical Bacterial Morphology.
Caccamo PD, Brun YV. Caccamo PD, et al. Trends Microbiol. 2018 Mar;26(3):191-208. doi: 10.1016/j.tim.2017.09.012. Epub 2017 Oct 19. Trends Microbiol. 2018. PMID: 29056293 Free PMC article. Review. - Mechanisms of bacterial morphogenesis: evolutionary cell biology approaches provide new insights.
Jiang C, Caccamo PD, Brun YV. Jiang C, et al. Bioessays. 2015 Apr;37(4):413-25. doi: 10.1002/bies.201400098. Epub 2015 Feb 9. Bioessays. 2015. PMID: 25664446 Free PMC article.
Cited by
- Constant surface area-to-volume ratio during cell growth as a design principle in mammalian cells.
Wu W, Lam AR, Suarez K, Smith GN, Duquette SM, Yu J, Mankus D, Bisher M, Lytton-Jean A, Manalis SR, Miettinen TP. Wu W, et al. bioRxiv [Preprint]. 2024 Jul 18:2024.07.02.601447. doi: 10.1101/2024.07.02.601447. bioRxiv. 2024. PMID: 39005340 Free PMC article. Preprint. - Bradymonabacteria, a novel bacterial predator group with versatile survival strategies in saline environments.
Mu DS, Wang S, Liang QY, Du ZZ, Tian R, Ouyang Y, Wang XP, Zhou A, Gong Y, Chen GJ, Van Nostrand J, Yang Y, Zhou J, Du ZJ. Mu DS, et al. Microbiome. 2020 Aug 31;8(1):126. doi: 10.1186/s40168-020-00902-0. Microbiome. 2020. PMID: 32867860 Free PMC article. - Diversity Takes Shape: Understanding the Mechanistic and Adaptive Basis of Bacterial Morphology.
Kysela DT, Randich AM, Caccamo PD, Brun YV. Kysela DT, et al. PLoS Biol. 2016 Oct 3;14(10):e1002565. doi: 10.1371/journal.pbio.1002565. eCollection 2016 Oct. PLoS Biol. 2016. PMID: 27695035 Free PMC article. - Geometric principles underlying the proliferation of a model cell system.
Wu LJ, Lee S, Park S, Eland LE, Wipat A, Holden S, Errington J. Wu LJ, et al. Nat Commun. 2020 Aug 18;11(1):4149. doi: 10.1038/s41467-020-17988-7. Nat Commun. 2020. PMID: 32811832 Free PMC article. - The exceptional form and function of the giant bacterium Ca. Epulopiscium viviparus revolves around its sodium motive force.
Sannino DR, Arroyo FA, Pepe-Ranney C, Chen W, Volland JM, Elisabeth NH, Angert ER. Sannino DR, et al. Proc Natl Acad Sci U S A. 2023 Dec 26;120(52):e2306160120. doi: 10.1073/pnas.2306160120. Epub 2023 Dec 18. Proc Natl Acad Sci U S A. 2023. PMID: 38109545 Free PMC article.
References
- Ackermann, M., S. C. Stearns, and U. Jenal. 2003. Senescence in a bacterium with asymmetric division. Science 300:1920. - PubMed
- Åkerlund, T., K. Nordström, and R. Bernander. 1993. Branched Escherichia coli cells. Mol. Microbiol. 10:849-858. - PubMed
- Allison, C., H. C. Lai, and C. Hughes. 1992. Co-ordinate expression of virulence genes during swarm-cell differentiation and population migration of Proteus mirabilis. Mol. Microbiol. 6:1583-1591. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources