One year of caloric restriction in humans: feasibility and effects on body composition and abdominal adipose tissue - PubMed (original) (raw)
Randomized Controlled Trial
One year of caloric restriction in humans: feasibility and effects on body composition and abdominal adipose tissue
Susan B Racette et al. J Gerontol A Biol Sci Med Sci. 2006 Sep.
Abstract
Background: Caloric restriction (CR) increases maximal life span in short-lived organisms, and its effects are being explored in nonhuman primates. The objectives of this study were to determine the feasibility of prolonged CR in nonobese adults and to compare the effects of CR- and exercise-induced weight loss on body composition and abdominal adiposity.
Methods: A randomized, controlled trial was conducted with 48 healthy, nonobese women and men, aged 57 +/- 1 (mean +/- standard error [SE]) years, with body mass index 27.3 +/- 0.3 kg/m2. Participants were randomly assigned to a 20% calorically-restricted diet (CR, n = 19), exercise designed to produce a similar energy deficit (EX, n = 19), or a healthy lifestyle control group (HL, n = 10) for 1 year. Assessments included weight, body composition by dual-energy x-ray absorptiometry, abdominal adipose tissue by magnetic resonance imaging, and energy intake by doubly labeled water.
Results: The average level of CR achieved by the CR group was 11.5 +/- 2.1%, and the EX group completed 59 +/- 6.7% of their prescribed exercise. Weight changes were greater (p <or=.0005) in the CR (-8.0 +/- 0.9 kg) and EX (-6.4 +/- 0.9) groups as compared to the HL group (-1.3 +/- 0.9 kg), corresponding to reductions of 10.7%, 8.4%, and 1.7% of baseline weights, respectively. Whole-body fat mass and visceral and subcutaneous abdominal adipose tissue decreased significantly (p <.005) and comparably in the CR and EX groups, but did not change in the HL group.
Conclusions: CR for 1 year was feasible, but the level of CR achieved was less than prescribed. CR and exercise were equally effective in reducing weight and adiposity.
Figures
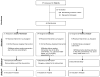
Figure 1
Consort diagram depicting the flow of participants through the Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy (CALERIE) trial at Washington University School of Medicine.
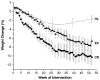
Figure 2
Body weight changes throughout the 1-year interventions. Symbols represent mean values (black circles, caloric restriction [CR]; black triangles, exercise [EX]; open circles, healthy lifestyle [HL]) ± standard error.
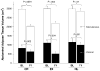
Figure 3
Visceral (black bars) and subcutaneous (white bars) abdominal adipose tissue by magnetic resonance imaging (MRI) at baseline and after 1 year of intervention. CR, caloric restriction; EX, exercise; HL, healthy lifestyle. Final values were adjusted for baseline differences between groups. Values of p represent the change within group from baseline (BL) to 1 year. For comparison between groups, the following contrasts were significant: CR versus HL for visceral adipose tissue (p = .05), CR versus HL for subcutaneous adipose tissue (p = .02), and EX versus HL for visceral adipose tissue (p = .01).
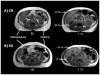
Figure 4
Magnetic resonance images of the abdomen at baseline (BL) and after the 1 year of caloric restriction (CR) (A) and exercise (EX) (B) interventions. Single-slice images are shown, although analyses were based on multislice volume estimates. The CR participant is a 54-year-old woman whose body weight decreased 12 kg (19%); the EX participant is a 60-year-old man whose weight decreased 16 kg (14%).
Similar articles
- Body-composition changes in the Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy (CALERIE)-2 study: a 2-y randomized controlled trial of calorie restriction in nonobese humans.
Das SK, Roberts SB, Bhapkar MV, Villareal DT, Fontana L, Martin CK, Racette SB, Fuss PJ, Kraus WE, Wong WW, Saltzman E, Pieper CF, Fielding RA, Schwartz AV, Ravussin E, Redman LM; CALERIE-2 Study Group. Das SK, et al. Am J Clin Nutr. 2017 Apr;105(4):913-927. doi: 10.3945/ajcn.116.137232. Epub 2017 Feb 22. Am J Clin Nutr. 2017. PMID: 28228420 Free PMC article. Clinical Trial. - Effect of calorie restriction with or without exercise on body composition and fat distribution.
Redman LM, Heilbronn LK, Martin CK, Alfonso A, Smith SR, Ravussin E; Pennington CALERIE Team. Redman LM, et al. J Clin Endocrinol Metab. 2007 Mar;92(3):865-72. doi: 10.1210/jc.2006-2184. Epub 2007 Jan 2. J Clin Endocrinol Metab. 2007. PMID: 17200169 Free PMC article. Clinical Trial. - Effect of exercise intensity on abdominal fat loss during calorie restriction in overweight and obese postmenopausal women: a randomized, controlled trial.
Nicklas BJ, Wang X, You T, Lyles MF, Demons J, Easter L, Berry MJ, Lenchik L, Carr JJ. Nicklas BJ, et al. Am J Clin Nutr. 2009 Apr;89(4):1043-52. doi: 10.3945/ajcn.2008.26938. Epub 2009 Feb 11. Am J Clin Nutr. 2009. PMID: 19211823 Free PMC article. Clinical Trial. - Visceral adiposity and inflammatory bowel disease.
Rowan CR, McManus J, Boland K, O'Toole A. Rowan CR, et al. Int J Colorectal Dis. 2021 Nov;36(11):2305-2319. doi: 10.1007/s00384-021-03968-w. Epub 2021 Jun 9. Int J Colorectal Dis. 2021. PMID: 34104989 Review. - Intermittent versus daily calorie restriction: which diet regimen is more effective for weight loss?
Varady KA. Varady KA. Obes Rev. 2011 Jul;12(7):e593-601. doi: 10.1111/j.1467-789X.2011.00873.x. Epub 2011 Mar 17. Obes Rev. 2011. PMID: 21410865 Review.
Cited by
- Calorie restriction reduces biomarkers of cellular senescence in humans.
Aversa Z, White TA, Heeren AA, Hulshizer CA, Saul D, Zhang X, Molina AJA, Redman LM, Martin CK, Racette SB, Huffman KM, Bhapkar M, Khosla S, Das SK, Fielding RA, Atkinson EJ, LeBrasseur NK. Aversa Z, et al. Aging Cell. 2024 Feb;23(2):e14038. doi: 10.1111/acel.14038. Epub 2023 Nov 14. Aging Cell. 2024. PMID: 37961856 Free PMC article. - Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trial.
Weiss EP, Racette SB, Villareal DT, Fontana L, Steger-May K, Schechtman KB, Klein S, Holloszy JO; Washington University School of Medicine CALERIE Group. Weiss EP, et al. Am J Clin Nutr. 2006 Nov;84(5):1033-42. doi: 10.1093/ajcn/84.5.1033. Am J Clin Nutr. 2006. PMID: 17093155 Free PMC article. Clinical Trial. - Dietary patterns and cardiometabolic health: Clinical evidence and mechanism.
Wang W, Liu Y, Li Y, Luo B, Lin Z, Chen K, Liu Y. Wang W, et al. MedComm (2020). 2023 Feb 5;4(1):e212. doi: 10.1002/mco2.212. eCollection 2023 Feb. MedComm (2020). 2023. PMID: 36776765 Free PMC article. Review. - Physiological Systems in Promoting Frailty.
Perazza LR, Brown-Borg HM, Thompson LV. Perazza LR, et al. Compr Physiol. 2022 Apr 26;12(3):3575-3620. doi: 10.1002/cphy.c210034. Compr Physiol. 2022. PMID: 35578945 Free PMC article. Review. - Does the method of weight loss effect long-term changes in weight, body composition or chronic disease risk factors in overweight or obese adults? A systematic review.
Washburn RA, Szabo AN, Lambourne K, Willis EA, Ptomey LT, Honas JJ, Herrmann SD, Donnelly JE. Washburn RA, et al. PLoS One. 2014 Oct 15;9(10):e109849. doi: 10.1371/journal.pone.0109849. eCollection 2014. PLoS One. 2014. PMID: 25333384 Free PMC article. Review.
References
- Mattison JA, Lane MA, Roth GS, Ingram DK. Calorie restriction in rhesus monkeys. Exp Gerontol. 2003;38:35–46. - PubMed
- Bodkin NL, Alexander TM, Ortmeyer HK, Johnson E, Hansen BC. Mortality and morbidity in laboratory-maintained Rhesus monkeys and effects of long-term dietary restriction. J Gerontol Biol Sci Med Sci. 2003;58A:212–219. - PubMed
- Walford RL, Mock D, Verdery R, MacCallum T. Calorie restriction in biosphere 2: alterations in physiologic, hematologic, hormonal, and biochemical parameters in humans restricted for a 2-year period. J Gerontol Biol Sci. 2002;57A:B211–B224. - PubMed
Publication types
MeSH terms
Grants and funding
- U01 AG020487/AG/NIA NIH HHS/United States
- RR00036/RR/NCRR NIH HHS/United States
- M01 RR000036/RR/NCRR NIH HHS/United States
- DK20579/DK/NIDDK NIH HHS/United States
- P30 DK056341/DK/NIDDK NIH HHS/United States
- 5-U01-AG20487/AG/NIA NIH HHS/United States
- P60 DK020579/DK/NIDDK NIH HHS/United States
- P30 DK020579/DK/NIDDK NIH HHS/United States
- T32 AG000078/AG/NIA NIH HHS/United States
- DK56341/DK/NIDDK NIH HHS/United States
- AG00078/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources