Influenza A virus NS1 protein binds p85beta and activates phosphatidylinositol-3-kinase signaling - PubMed (original) (raw)
Influenza A virus NS1 protein binds p85beta and activates phosphatidylinositol-3-kinase signaling
Benjamin G Hale et al. Proc Natl Acad Sci U S A. 2006.
Abstract
Influenza A virus NS1 is a multifunctional protein, and in virus-infected cells NS1 modulates a number of host-cell processes by interacting with cellular factors. Here, we report that NS1 binds directly to p85beta, a regulatory subunit of phosphatidylinositol-3-kinase (PI3K), but not to the related p85alpha subunit. Activation of PI3K in influenza virus-infected cells depended on genome replication, and showed kinetics that correlated with NS1 expression. Additionally, it was found that expression of NS1 alone was sufficient to constitutively activate PI3K, causing the phosphorylation of a downstream mediator of PI3K signal transduction, Akt. Mutational analysis of a potential SH2-binding motif within NS1 indicated that the highly conserved tyrosine at residue 89 is important for both the interaction with p85beta, and the activation of PI3K. A mutant influenza virus (A/Udorn/72) expressing NS1 with the Y89F amino acid substitution exhibited a small-plaque phenotype, and grew more slowly in tissue culture than WT virus. These data suggest that activation of PI3K signaling in influenza A virus-infected cells is important for efficient virus replication.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
Fig. 1.
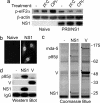
Characterization of an NS1-expressing HEp2 cell line. (a) PKR activation in naïve or PR8/NS1-expressing HEp2 cells was assessed by the induction of phospho-eIF2α (Ser-51) in response to mock-transfection (−), transfection of poly I:C (pI:C), or RNA virus infection (multiplicity of infection of 5 PFU per cell, CPI−). Twenty hours after treatment, monolayers were harvested, and protein lysates were separated by SDS/PAGE followed by transfer to PVDF membrane. Phospho-eIF2α (Ser-51) and β-actin were detected by using specific antibodies. NS1 expression was detected by using an anti-V5 mAb. (b) Predominant nuclear localization of V5-tagged NS1 in NS1-expressing HEp2 cells was confirmed by indirect-immunofluorescence using an anti-V5 mAb. (c) p85β interacts with NS1. Soluble antigen extracts from ≈2 × 107 naïve, NS1-, or SeV/V-expressing HEp2 cells were immunoprecipitated with anti-V5 mAb cross-linked to protein G Sepharose. Precipitated proteins were separated by electrophoresis through 4–12% polyacrylamide gradient gels and visualized by Coomassie blue staining. Polypeptide bands denoted by an asterisk were excised and identified by mass spectrometry. Molecular mass markers (in kilodaltons) are indicated on the right. (d) Immunoprecipitates were prepared as in c and separated by electrophoresis followed by transfer to PVDF membrane. p85β was detected by using a specific mAb. Anti-V5 mAb was used to detect V5-tagged NS1 and SeV/V.
Fig. 2.
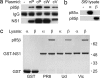
The NS1 proteins of several influenza virus strains bind efficiently to p85β but not p85α. (a) 293T cells were transfected for 48 h with empty vector (−), a plasmid expressing the N-terminal V5-tagged NS1 protein of PR8 (nP), or plasmids expressing the C-terminal V5-tagged NS1 proteins of PR8 (cP), WSN (cW), or Vic (cV). Soluble antigen extracts were immunoprecipitated with anti-V5 antibody and precipitates separated by SDS/PAGE followed by transfer to PVDF membrane. Endogenous p85β was detected by using a specific mAb. Anti-V5 mAb was used to detect V5-tagged NS1 proteins. (b) Sf9 cells were infected (or mock, −) with recombinant baculoviruses expressing either p85α or p85β. Equal amounts of soluble lysate were separated by electrophoresis, transferred to PVDF membrane, and probed for p85α or p85β expression using specific mAbs. (c) Equal amounts of soluble Sf9 cell lysate from b were mixed with GST or GST-NS1 (PR8, Ud, or Vic) immobilized onto glutathione-agarose beads. After washing, protein complexes were dissociated from the beads and separated by SDS/PAGE through 4–12% polyacrylamide gradient gels. Polypeptides were stained with Coomassie blue, and protein identification was confirmed by mass spectrometry. Molecular mass markers (in kilodaltons) are indicated on the right.
Fig. 3.
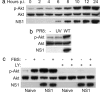
NS1 induces the phosphorylation of Akt (Ser-473) in a PI3K-dependent manner. (a) Time course of Akt phosphorylation at Ser-473 after influenza A virus (PR8) infection. Confluent serum-starved monolayers of 1321N1 cells were infected with influenza virus at a multiplicity of infection of 5 PFU per cell, and total cell lysates were harvested at various times p.i. Lysates were separated by SDS/PAGE followed by transfer to PVDF membrane. Phospho-Akt (Ser-473) was detected by using a specific mAb. Total Akt was detected by using a pAb and acted as a loading control. NS1 expression was detected by using a specific pAb. (b) UV-inactivated influenza virus (PR8) does not induce Akt phosphorylation. Serum-starved 1321N1 monolayers were infected (or mock, −) as in a with either untreated (WT) or UV-inactivated (UV) virus. Lysates were prepared 20 h p.i. and analyzed as in a. (c) Serum-starved naïve or PR8/NS1-expressing 1321N1 monolayers were treated (or mock) with 5% FBS for 1 h. In a duplicate experiment, cells were also treated with 25 μM LY294002 (a specific PI3K inhibitor; LY). Cell lysates were analyzed as in a. NS1 was detected by using an anti-V5 mAb.
Fig. 4.
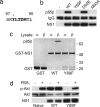
Binding of p85β and activation of PI3K requires residues from a Y_XX_M-like motif in NS1. (a) Amino acid sequence of PR8/NS1 residues 87–95. The putative Y_XX_M-like motif is shown in bold. Y89 (denoted by an asterisk) is totally conserved among all NS1 proteins of influenza A strains sequenced to date. (b) Binding of NS1 to p85β requires Y89 and M93. 293T cells were transfected for 48 h with either empty vector (−), a plasmid expressing WT V5-tagged PR8/NS1, or plasmids expressing V5-tagged PR8/NS1 proteins with single amino acid substitutions (Y89F, D92E, or M93A). Soluble antigen extracts were immunoprecipitated with anti-V5 antibody and precipitates separated by SDS/PAGE followed by transfer to PVDF membrane. p85β was detected by using a specific mAb. Anti-V5 mAb was used to detect the V5-tagged NS1 mutants. (c) WT GST-PR8/NS1 and GST-PR8/NS1 with the Y89F mutation were used as bait to precipitate p85α or p85β from baculovirus-infected Sf9 cell lysates. Protein complexes were analyzed as for Fig. 2_c_. (d) Expression of NS1 with the single amino acid substitution Y89F does not induce the phosphorylation of Akt at Ser-473. Serum-starved naïve, WT PR8/NS1-expressing (WT), or mutant PR8/NS1-expressing (Y89F) 1321N1 monolayers were treated (or mock-treated) with 5% FBS for 1 h. Cell lysates were separated by SDS/PAGE followed by transfer to PVDF membrane. Phospho-Akt (Ser-473) was detected by using a specific mAb. Total Akt was detected by using a pAb and acted as a loading control. NS1 (WT and Y89F) was detected by using an anti-V5 mAb.
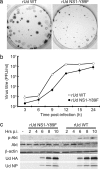
Fig. 5.
Characterization of a recombinant Ud virus (rUd) expressing NS1 with the Y89F amino acid substitution. (a) rUd NS1-Y89F forms smaller plaques than WT rUd virus. Confluent MDCK monolayers were infected at equal multiplicities of infection with either WT rUd or rUd NS1-Y89F. Plaques were fixed 4 days p.i. and immunostained with goat anti-A/Udorn/72 virus serum. (b) Single-step growth analysis of WT rUd (open circles) and rUd NS1-Y89F (filled circles). Recombinant Ud viruses were used to infect MDCK cell monolayers (in the absence of TPCK-trypsin) at a multiplicity of infection of 3 PFU per cell. Virus-containing supernatants were harvested at various times p.i. and titrated by plaque assay. The mean titers from three independent experiments are shown. Error bars represent standard deviation. (c) Phosphorylation of Akt in response to infection with either WT rUd or rUd NS1-Y89F viruses. Confluent serum-starved monolayers of 1321N1 cells were infected at a multiplicity of infection of 5 PFU per cell, and total cell lysates were harvested at various times p.i. Lysates were separated by SDS/PAGE followed by transfer to PVDF membrane. Phospho-Akt (Ser-473) was detected by using a specific mAb. Total Akt was detected by using a pAb, and β-actin acted as a loading control. Ud hemagglutination and NP were detected by using goat anti-Ud serum.
Similar articles
- SH3 binding motif 1 in influenza A virus NS1 protein is essential for PI3K/Akt signaling pathway activation.
Shin YK, Li Y, Liu Q, Anderson DH, Babiuk LA, Zhou Y. Shin YK, et al. J Virol. 2007 Dec;81(23):12730-9. doi: 10.1128/JVI.01427-07. Epub 2007 Sep 19. J Virol. 2007. PMID: 17881440 Free PMC article. - Influenza A virus NS1 protein activates the phosphatidylinositol 3-kinase (PI3K)/Akt pathway by direct interaction with the p85 subunit of PI3K.
Shin YK, Liu Q, Tikoo SK, Babiuk LA, Zhou Y. Shin YK, et al. J Gen Virol. 2007 Jan;88(Pt 1):13-18. doi: 10.1099/vir.0.82419-0. J Gen Virol. 2007. PMID: 17170431 - Influenza A virus NS1 protein activates the PI3K/Akt pathway to mediate antiapoptotic signaling responses.
Ehrhardt C, Wolff T, Pleschka S, Planz O, Beermann W, Bode JG, Schmolke M, Ludwig S. Ehrhardt C, et al. J Virol. 2007 Apr;81(7):3058-67. doi: 10.1128/JVI.02082-06. Epub 2007 Jan 17. J Virol. 2007. PMID: 17229704 Free PMC article. - A new player in a deadly game: influenza viruses and the PI3K/Akt signalling pathway.
Ehrhardt C, Ludwig S. Ehrhardt C, et al. Cell Microbiol. 2009 Jun;11(6):863-71. doi: 10.1111/j.1462-5822.2009.01309.x. Epub 2009 Mar 12. Cell Microbiol. 2009. PMID: 19290913 Free PMC article. Review. - Influenza A viruses and PI3K: are there time, place and manner restrictions?
Ayllon J, García-Sastre A, Hale BG. Ayllon J, et al. Virulence. 2012 Jul 1;3(4):411-4. doi: 10.4161/viru.20932. Epub 2012 Jun 22. Virulence. 2012. PMID: 22722241 Free PMC article. Review. No abstract available.
Cited by
- Synergistic effect of the PDZ and p85β-binding domains of the NS1 protein on virulence of an avian H5N1 influenza A virus.
Fan S, Macken CA, Li C, Ozawa M, Goto H, Iswahyudi NF, Nidom CA, Chen H, Neumann G, Kawaoka Y. Fan S, et al. J Virol. 2013 May;87(9):4861-71. doi: 10.1128/JVI.02608-12. Epub 2013 Feb 13. J Virol. 2013. PMID: 23408626 Free PMC article. - Double-stranded RNA-induced activation of activating protein-1 promoter is differentially regulated by the non-structural protein 1 of avian influenza A viruses.
Munir M, Zohari S, Belák S, Berg M. Munir M, et al. Viral Immunol. 2012 Feb;25(1):79-85. doi: 10.1089/vim.2011.0059. Epub 2012 Jan 12. Viral Immunol. 2012. PMID: 22239235 Free PMC article. - A Second RNA-Binding Site in the NS1 Protein of Influenza B Virus.
Ma LC, Guan R, Hamilton K, Aramini JM, Mao L, Wang S, Krug RM, Montelione GT. Ma LC, et al. Structure. 2016 Sep 6;24(9):1562-72. doi: 10.1016/j.str.2016.07.001. Epub 2016 Aug 18. Structure. 2016. PMID: 27545620 Free PMC article. - Inhibition of Phosphatidylinositol 3-Kinase by Pictilisib Blocks Influenza Virus Propagation in Cells and in Lungs of Infected Mice.
Deinhardt-Emmer S, Jäckel L, Häring C, Böttcher S, Wilden JJ, Glück B, Heller R, Schmidtke M, Koch M, Löffler B, Ludwig S, Ehrhardt C. Deinhardt-Emmer S, et al. Biomolecules. 2021 May 29;11(6):808. doi: 10.3390/biom11060808. Biomolecules. 2021. PMID: 34072389 Free PMC article. - Heterologous SH3-p85beta inhibits influenza A virus replication.
Zhang DG, Li WZ, Wang GF, Su Y, Zeng J, Zhang C, Zeng XX, Chen XX, Xu YX, Li KS. Zhang DG, et al. Virol J. 2010 Jul 23;7:170. doi: 10.1186/1743-422X-7-170. Virol J. 2010. PMID: 20653952 Free PMC article.
References
- Wright PF, Webster RG. In: Fields Virology. 4th Ed. Knipe DM, Howley PM, editors. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 1533–1579.
- Noah DL, Krug RM. Adv Virus Res. 2005;65:121–145. - PubMed
- Krug RM, Yuan W, Noah DL, Latham AG. Virology. 2003;309:181–189. - PubMed
- Garcia-Sastre A. Virology. 2001;279:375–384. - PubMed
- Garcia-Sastre A, Egorov A, Matassov D, Brandt S, Levy DE, Durbin JE, Palese P, Muster T. Virology. 1998;252:324–330. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases