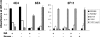Role of lgtC in resistance of nontypeable Haemophilus influenzae strain R2866 to human serum - PubMed (original) (raw)
. 2006 Nov;74(11):6226-35.
doi: 10.1128/IAI.00722-06. Epub 2006 Sep 11.
Simon Allen, Derek K Ho, Paul J Bonthuis, Justin Jarisch, Kevin L Nelson, David L Tsao, William C T Unrath, Michael E Watson Jr, Bradford W Gibson, Michael A Apicella, Arnold L Smith
Affiliations
- PMID: 16966407
- PMCID: PMC1695526
- DOI: 10.1128/IAI.00722-06
Role of lgtC in resistance of nontypeable Haemophilus influenzae strain R2866 to human serum
Alice L Erwin et al. Infect Immun. 2006 Nov.
Erratum in
- Infect Immun. 2006 Dec;74(12):7047. Bonthius, Paul J [corrected to Bonthuis, Paul J]
Abstract
We are investigating a nontypeable Haemophilus influenzae (NTHI) strain, R2866, isolated from a child with meningitis. R2866 is unusually resistant to killing by normal human serum. The serum 50% inhibitory concentration (IC50) for this strain is 18%, approaching that of encapsulated H. influenzae. R3392 is a derivative of R2866 that was found to have increased sensitivity to human serum (IC50, 1.5%). Analysis of tetrameric repeat regions within lipooligosaccharide (LOS) biosynthetic genes in both strains indicated that the glycosyltransferase gene lgtC was out of frame ("off") in most colonies of R3392 but in frame with its start codon ("on") in most colonies of the parent. We sought antigenic and biochemical evidence for modification of the LOS structure. In a whole-cell enzyme-linked immunosorbent assay, strain R3392 displayed reduced binding of the Galalpha1,4Gal-specific monoclonal antibody 4C4. Mass spectrometry analysis of LOS from strain R2866 indicated that the primary oligosaccharide glycoform contained four heptose and four hexose residues, while that of R3392 contained four heptose and three hexose residues. We conclude that the R2866 lgtC gene encodes a galactosyltransferase involved in synthesis of the 4C4 epitope, as in other strains, and that expression of lgtC is associated with the high-level serum resistance that has been observed for this strain. This is the first description of the genetic basis of high-level serum resistance in NTHI, as well as the first description of LOS composition in an NTHI strain for which the complete genome sequence has been determined.
Figures
FIG. 1.
IC50 of normal human serum for cultures derived from individual colonies of R2866, R3392, R3521, and R3522. DNA preparations from the same colonies were used for the data shown in Table 3. For strain R3392, data for _lic3A-_on and _lic3A-_off colonies are plotted separately. All R3392 colonies are lgtC off, with the exception of the single colony with an IC50 of 15%, which is lgtC on. For R3521, all colonies are lgtC on. Serum IC50 was significantly higher for colonies of R2866 than for colonies of R3392 (P < 0.0001); serum IC50 was significantly higher for colonies of R3521 than for colonies of R3522 (P < 0.0001).
FIG. 2.
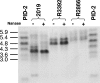
SDS-PAGE analysis of nontypeable H. influenzae treated (+) or not treated (−) with neuraminidase (Nanase). PID-2, reference LOS from N. gonorrhoeae; 2019, reference strain, included as a neuraminidase control. Although the R2866 lanes are underloaded relative to the other samples, it is apparent that R3392 LOS has greater mobility than that of R2866 and that neuraminidase treatment does not affect the mobility of the LOS prepared from either of these two strains.
FIG. 3.
Whole-cell ELISA of nontypeable H. influenzae strains 2019, R3392, and R2866 with monoclonal antibodies 6E4, 3F11, and 4C4. Bacteria were grown in the presence or absence of neuraminic acid (NA), and selected wells were treated with neuraminidase (Nanase) before exposure to antibody. Strains 1291 and 1291 pgm were used as positive and negative controls, respectively, for 3F11 reactivity. The absorbance at 405 nm indicates the relative amount of antigen exposed for antibody detection. Representative data are shown and expressed as means of six replicates ± standard deviation.
FIG. 4.
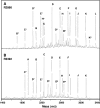
Negative-ion MALDI time-of-flight mass spectra of dephosphorylated _O_-LOS from H. influenzae stains R2866 (A) and R3392 (B). Note the differences between the two panels in peaks C (representing a glycoform with three hexoses and four heptoses) and D (four hexoses and four heptoses). See Table 4 for molecular weights and proposed compositions. An asterisk indicates the loss of half the lipid A moiety from the O-deacylated LOS, and p indicates the presence of a phosphate moiety.
FIG. 5.
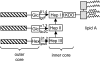
Schematized structure for H. influenzae LOS, based on data summarized in reference . The three hatched bars represent glycosyl chains (one or more residues) that may be present. Nonsugar substituents such as phosphorylcholine, acetate, glycine, and phosphoethanolamine are not shown. Hep, heptose; Glc, glucose; Hex, hexose.
Similar articles
- Infant rat infection modifies phenotypic properties of an invasive nontypeable Haemophilus influenzae.
Tsao D, Nelson KL, Kim D, Smith AL. Tsao D, et al. Microbes Infect. 2012 Jun;14(6):509-16. doi: 10.1016/j.micinf.2011.12.010. Epub 2011 Dec 27. Microbes Infect. 2012. PMID: 22222846 Free PMC article. - lgtC expression modulates resistance to C4b deposition on an invasive nontypeable Haemophilus influenzae.
Ho DK, Ram S, Nelson KL, Bonthuis PJ, Smith AL. Ho DK, et al. J Immunol. 2007 Jan 15;178(2):1002-12. doi: 10.4049/jimmunol.178.2.1002. J Immunol. 2007. PMID: 17202363 - The modA10 phasevarion of nontypeable Haemophilus influenzae R2866 regulates multiple virulence-associated traits.
VanWagoner TM, Atack JM, Nelson KL, Smith HK, Fox KL, Jennings MP, Stull TL, Smith AL. VanWagoner TM, et al. Microb Pathog. 2016 Mar;92:60-67. doi: 10.1016/j.micpath.2015.12.006. Epub 2015 Dec 21. Microb Pathog. 2016. PMID: 26718097 Free PMC article. - Naturally occurring bactericidal antibodies specific for Haemophilus influenzae lipooligosaccharide are present in healthy adult individuals.
Choi J, Nix EB, Gaultier GN, Cox AD, McCready W, Ulanova M. Choi J, et al. Vaccine. 2015 Apr 15;33(16):1941-7. doi: 10.1016/j.vaccine.2015.02.060. Epub 2015 Mar 1. Vaccine. 2015. PMID: 25738817 - Modified lipooligosaccharide structure protects nontypeable Haemophilus influenzae from IgM-mediated complement killing in experimental otitis media.
Langereis JD, Stol K, Schweda EK, Twelkmeyer B, Bootsma HJ, de Vries SP, Burghout P, Diavatopoulos DA, Hermans PW. Langereis JD, et al. mBio. 2012 Jul 3;3(4):e00079-12. doi: 10.1128/mBio.00079-12. Print 2012. mBio. 2012. PMID: 22761391 Free PMC article.
Cited by
- Identification of non-substrate-like glycosyltransferase inhibitors from library screening: pitfalls & hits.
Ema M, Xu Y, Gehrke S, Wagner GK. Ema M, et al. Medchemcomm. 2017 Nov 29;9(1):131-137. doi: 10.1039/c7md00550d. eCollection 2018 Jan 1. Medchemcomm. 2017. PMID: 30108907 Free PMC article. - Association of IS1016 with the hia adhesin gene and biotypes V and I in invasive nontypeable Haemophilus influenzae.
Satola SW, Napier B, Farley MM. Satola SW, et al. Infect Immun. 2008 Nov;76(11):5221-7. doi: 10.1128/IAI.00672-08. Epub 2008 Sep 15. Infect Immun. 2008. PMID: 18794287 Free PMC article. - Molecular basis of increased serum resistance among pulmonary isolates of non-typeable Haemophilus influenzae.
Nakamura S, Shchepetov M, Dalia AB, Clark SE, Murphy TF, Sethi S, Gilsdorf JR, Smith AL, Weiser JN. Nakamura S, et al. PLoS Pathog. 2011 Jan 6;7(1):e1001247. doi: 10.1371/journal.ppat.1001247. PLoS Pathog. 2011. PMID: 21253576 Free PMC article. - Infant rat infection modifies phenotypic properties of an invasive nontypeable Haemophilus influenzae.
Tsao D, Nelson KL, Kim D, Smith AL. Tsao D, et al. Microbes Infect. 2012 Jun;14(6):509-16. doi: 10.1016/j.micinf.2011.12.010. Epub 2011 Dec 27. Microbes Infect. 2012. PMID: 22222846 Free PMC article. - LuxS promotes biofilm maturation and persistence of nontypeable haemophilus influenzae in vivo via modulation of lipooligosaccharides on the bacterial surface.
Armbruster CE, Hong W, Pang B, Dew KE, Juneau RA, Byrd MS, Love CF, Kock ND, Swords WE. Armbruster CE, et al. Infect Immun. 2009 Sep;77(9):4081-91. doi: 10.1128/IAI.00320-09. Epub 2009 Jun 29. Infect Immun. 2009. PMID: 19564381 Free PMC article.
References
- Abdillahi, H., and J. T. Poolman. 1988. Typing of group-B Neisseria meningitidis with monoclonal antibodies in the whole-cell ELISA. J. Med. Microbiol. 26:177-180. - PubMed
- Bouchet, V., D. W. Hood, J. Li, J. R. Brisson, G. A. Randle, A. Martin, Z. Li, R. Goldstein, E. K. Schweda, S. I. Pelton, J. C. Richards, and E. R. Moxon. 2003. Host-derived sialic acid is incorporated into Haemophilus influenzae lipopolysaccharide and is a major virulence factor in experimental otitis media. Proc. Natl. Acad. Sci. USA 100:8898-8903. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DC005833/DC/NIDCD NIH HHS/United States
- AI 46512/AI/NIAID NIH HHS/United States
- R37 AI024616/AI/NIAID NIH HHS/United States
- AI 24616/AI/NIAID NIH HHS/United States
- R01 AI046512/AI/NIAID NIH HHS/United States
- AI 44002/AI/NIAID NIH HHS/United States
- R01 AI024616/AI/NIAID NIH HHS/United States
- DC 005833/DC/NIDCD NIH HHS/United States
- R01 AI044002/AI/NIAID NIH HHS/United States
- R56 AI024616/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases