Progesterone blocks estrogen-induced DNA synthesis through the inhibition of replication licensing - PubMed (original) (raw)
Progesterone blocks estrogen-induced DNA synthesis through the inhibition of replication licensing
Haiyan Pan et al. Proc Natl Acad Sci U S A. 2006.
Abstract
In the uterus, progesterone (P4) acts early in G1 as a physiological inhibitor of estradiol-17beta (E2)-induced epithelial cell proliferation. Gene expression profiling of uterine epithelial cell RNA isolated 3 h after hormonal treatment of ovariectomized mice revealed the co-coordinate down-regulation by P4 of >20 genes whose functions are associated with DNA replication. This group included all of the minichromosome maintenance (MCM) proteins that are required for DNA replication licensing. E2 regulated loading of these MCM proteins onto chromatin in parallel with its induction of DNA synthesis. E2 caused this chromatin loading by retention of MCM proteins in the nucleus and through the induction of the loading factor Cdt1, which is necessary for the MCM heterohexamer to bind to the origin of DNA replication. P4 dramatically reduced the binding of the MCMs to chromatin by a number of mechanisms. First, MCM mRNA and protein abundance was down-regulated. Second, P4 inhibited the E2 induction of Cdt1. Third, P4 treatment sequestered the normally nuclear MCM proteins into the cytoplasm. This reduced MCM binding resulted in the complete inhibition of E2-induced DNA synthesis by P4. These data reveal mechanisms not only for female sex steroid hormone action but also in the regulation of DNA replication licensing.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
Fig. 1.
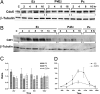
Hormonal regulation of Mcm expression. (A) Validation of cDNA microarray data by QRT-PCR by using the gene-specific primers shown in Table 3, which is published as supporting information on the PNAS web site. The y axis shows the amplitude of down-regulation determined by Microarray or QRT-PCR of the genes shown on the x axis. Data shown are the mean ± SD of three experiments and show significant down-regulation by P4 compared with E2 treatment. (B) Synergistic down-regulation of MCM2, MCM3, and MCM4 protein concentration by E2 and P4. Ovariectomized mice were killed at the times shown after the different hormone treatments as indicated. Equivalent amounts (60 μg) of protein isolated from the total epithelial cell lysates were separated by SDS/PAGE, blotted onto Nylon membranes, and probed with the antibodies against the proteins listed on the side. Detection of β-tubulin with an anti-β-tubulin antibody was used as a protein loading control. The Western blots shown are representative of those obtained from three independent experiments. (C and D) Densitometric analysis of the expression of MCM2 (C) and MCM3 (D) after the various treatments shown. Data shown are the mean ± SD of three experiments.
Fig. 2.
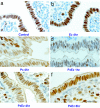
MCM3 localization is controlled by E2 and P4 in the mouse uterine epithelium. Shown is immunostaining for MCM3 of uterine transverse sections isolated at the indicated times after the different hormonal treatments. (a) Control. (b) Fifty nanograms of E2 at 11 h. (c) One milligram of P4 for 4 days. (d and e) One milligram of P4 for 4 days and killed at 1 h (d) and 4 h (e) after 50 ng of E2 on the fourth day. (f) Inhibition of the IHC signal by a competitive MCM3 peptide. Brown indicates positive staining, and the columnar cells are the luminal epithelium. (Scale bar: 50 μm.)
Fig. 3.
P4 transiently excludes MCM6 from the nucleus. Shown is immunostaining for MCM6 of uterine transverse sections isolated at the indicated times after the different hormonal treatments. (a) Control. (b) Fifty nanograms of E2 at 4 h. (c) One milligram of P4 for 4 days. (d_–_f) One milligram of P4 for 4 days and killed 1 h (d), 4 h (e), and 8 h (f) after 50 ng of E2 on the fourth day. (Scale bar: 50 μm.)
Fig. 4.
P4 inhibits the E2-induced chromatin binding of MCM complexes in the mouse uterine epithelium. Uterine luminal epithelial cells were purified at indicated times after treatment with vehicle alone (C), E2, P4, or P4E2 as described. Chromatin-bound insoluble proteins were separated from total epithelial lysates as described in Materials and Methods. Equal amounts (30 μg) of protein were separated by SDS/PAGE and blotted onto nylon membranes. These blots were probed as indicated with antibodies to MCM2, MCM3, and lamin A/C. Lamin A/C served as the loading control for the chromatin-bound MCM2 and MCM3 proteins. Shown are representative Western blots from three independent experiments.
Fig. 5.
P4 and E2 differentially regulate the two DNA replication initiation factors Cdc6 and Cdt1 in the uterine epithelium. Shown are representative Western blots from three independent experiments of total uterine luminal epithelial cell extracts prepared at various time points after hormonal treatments as indicated and probed with anti-Cdc6 antibody (A) and anti-Cdt1 antibody (B). Detection of β-tubulin was used as the loading control. (C and D) Densitometric analysis of the expression of Cdc6 (C) and Cdt1 (D) at different time points after the hormonal treatments as indicated. Data shown are the mean ± SD of three experiments.
Similar articles
- KLF15 negatively regulates estrogen-induced epithelial cell proliferation by inhibition of DNA replication licensing.
Ray S, Pollard JW. Ray S, et al. Proc Natl Acad Sci U S A. 2012 May 22;109(21):E1334-43. doi: 10.1073/pnas.1118515109. Epub 2012 Apr 26. Proc Natl Acad Sci U S A. 2012. PMID: 22538816 Free PMC article. - Progesterone inhibits the estrogen-induced phosphoinositide 3-kinase-->AKT-->GSK-3beta-->cyclin D1-->pRB pathway to block uterine epithelial cell proliferation.
Chen B, Pan H, Zhu L, Deng Y, Pollard JW. Chen B, et al. Mol Endocrinol. 2005 Aug;19(8):1978-90. doi: 10.1210/me.2004-0274. Epub 2005 Apr 21. Mol Endocrinol. 2005. PMID: 15845746 - Characterization of conserved arginine residues on Cdt1 that affect licensing activity and interaction with Geminin or Mcm complex.
You Z, Ode KL, Shindo M, Takisawa H, Masai H. You Z, et al. Cell Cycle. 2016 May 2;15(9):1213-26. doi: 10.1080/15384101.2015.1106652. Cell Cycle. 2016. PMID: 26940553 Free PMC article. - The MCM complex: its role in DNA replication and implications for cancer therapy.
Lei M. Lei M. Curr Cancer Drug Targets. 2005 Aug;5(5):365-80. doi: 10.2174/1568009054629654. Curr Cancer Drug Targets. 2005. PMID: 16101384 Review. - Control of DNA replication licensing in a cell cycle.
Nishitani H, Lygerou Z. Nishitani H, et al. Genes Cells. 2002 Jun;7(6):523-34. doi: 10.1046/j.1365-2443.2002.00544.x. Genes Cells. 2002. PMID: 12059957 Review.
Cited by
- Enhancing endometrial receptivity: the roles of human chorionic gonadotropin in autophagy and apoptosis regulation in endometrial stromal cells.
Wang B, Gao M, Yao Y, Shen H, Li H, Sun J, Wang L, Zhang X. Wang B, et al. Reprod Biol Endocrinol. 2024 Apr 4;22(1):37. doi: 10.1186/s12958-024-01205-x. Reprod Biol Endocrinol. 2024. PMID: 38576003 Free PMC article. - MCM6 Inhibits Decidualization via Cross-Talking with ERK Pathway in Human Endometrial Stromal Cells.
Jiang Y, Xue Y, Yuan X, Ye S, Liu M, Shi Y, Zhou H. Jiang Y, et al. Reprod Sci. 2024 Jul;31(7):1915-1923. doi: 10.1007/s43032-024-01463-5. Epub 2024 Feb 12. Reprod Sci. 2024. PMID: 38347378 - Progesterone Resistance in Endometriosis: Current Evidence and Putative Mechanisms.
Zhang P, Wang G. Zhang P, et al. Int J Mol Sci. 2023 Apr 10;24(8):6992. doi: 10.3390/ijms24086992. Int J Mol Sci. 2023. PMID: 37108154 Free PMC article. Review. - Loss of MIG-6 results in endometrial progesterone resistance via ERBB2.
Yoo JY, Kim TH, Shin JH, Marquardt RM, Müller U, Fazleabas AT, Young SL, Lessey BA, Yoon HG, Jeong JW. Yoo JY, et al. Nat Commun. 2022 Mar 1;13(1):1101. doi: 10.1038/s41467-022-28608-x. Nat Commun. 2022. PMID: 35232969 Free PMC article. - The Flavonoid Baicalein Negatively Regulates Progesterone Target Genes in the Uterus in Vivo.
Li K, Diakite D, Austin J, Lee JH, Lantvit DD, Murphy BT, Burdette JE. Li K, et al. J Nat Prod. 2022 Jan 28;85(1):237-247. doi: 10.1021/acs.jnatprod.1c01008. Epub 2021 Dec 22. J Nat Prod. 2022. PMID: 34935393 Free PMC article.
References
- Tong W, Pollard JW. In: The Endometrium. Glasser SR, Aplin JD, Giudice LC, Tabibzadeh S, editors. London: Taylor & Francis; 2002. pp. 94–109.
- Martin L, Finn CA, Trinder G. Endocrinol. 1973;56:303–307. - PubMed
- Finn CA, Martin L. J Endocrinol. 1969;45:57–65. - PubMed
- Martin L, Das RM, Finn CA. J Endocrinol. 1973;57:549–554. - PubMed
- Martin L, Finn CA, Trinder G. J Endocrinol. 1973;56:133–144. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources