Estrogen receptor protein interaction with phosphatidylinositol 3-kinase leads to activation of phosphorylated Akt and extracellular signal-regulated kinase 1/2 in the same population of cortical neurons: a unified mechanism of estrogen action - PubMed (original) (raw)
Estrogen receptor protein interaction with phosphatidylinositol 3-kinase leads to activation of phosphorylated Akt and extracellular signal-regulated kinase 1/2 in the same population of cortical neurons: a unified mechanism of estrogen action
Paolo Mannella et al. J Neurosci. 2006.
Abstract
17Beta-estradiol (E2)-induced neuroprotection is dependent on mitogen-activated protein kinase (MAPK) and phosphatidylinositol-3-kinase (PI3K) signaling cascades. We sought to determine whether E2 neuroprotective mechanisms are mediated by a unified signaling cascade activated by estrogen receptor (ER)-PI3K interaction within the same population of neurons or whether E2 activation of extracellular signal-regulated kinase 1/2 (ERK1/2) and Akt are independent signaling events in different neuronal populations. Immunoprecipitation of E2-treated cortical neurons was conducted to determine a protein-protein interaction between ER and the PI3K regulatory subunit p85. Subsequently, cortical neurons were treated with E2 alone or in presence of MAPK inhibitors or PI3K inhibitors. Results of these analyses indicated a protein-protein interaction between ER and p85 that was time-dependent and consistent with the temporal profile for generation of Akt (pAkt) and ERK1/2 phosphorylation (pERK1/2). E2-induced phosphorylation of Akt, was first apparent at 10 min and maximal at 30 min. Simultaneously, E2-induced pERK1/2 was first apparent at 5-10 min and maximal at 30 min. Inhibition of PI3K completely blocked E2 activation of pAkt at 10 and 30 min and blocked E2 activation of ERK1/2 at 10 min, which revealed a PI3K-independent activation of ERK at 30 min. Double immunocytochemical labeling for pERK1/2 and pAkt demonstrated that E2 induced both signaling pathways in the same neurons. These results indicate a unified signaling mechanism for rapid E2 action that leads to the coordinated activation of both pERK1/2 and pAkt in the same population of neurons. Implications of these results for understanding estrogen mechanism of action in neurons and therapeutic development are considered.
Figures
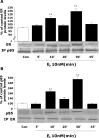
Figure 1.
Dynamic protein–protein interaction between estrogen receptor and PI3K regulatory subunit p85. Rat cortical neurons were exposed to E2 (10 n
m
) for specific time intervals (0, 5, 10, 20, 30, and 45 min), and lysates were immunoprecipitated (IP) with either anti-p85, the regulatory subunit of PI3K, or ER (clone TE111.5D11), which principally detects ERα but can partially recognize ERβ. A, Lysates immunoprecipitated with anti-p85, the regulatory subunit of PI3K, were subsequently immunoblotted for detection of ER. A dynamic pattern of p85–ER interaction was apparent in which significant immunoreactivity of ER occurred at 10 and 30 min, with intervening uncoupling at 20 and 45 min. B, To confirm these findings, the corollary experiment was performed. Cortical neuron lysates were immunoprecipitated with anti-ER and subsequently immunoblotted with anti- p85. Evidence for an ER protein interaction with p85 occurred at 10 and 30 min, with maximal complexing of the proteins at the 30 min time point. The uncoupling of proteins was apparent at 20 and 45 min. Data are from a single experiment and are representative of three independent experiments. *p < 0.05 versus control neurons; **p < 0.01 versus control neurons. Con, Control.
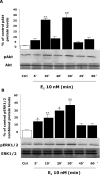
Figure 2.
E2 induces Akt and ERK1/2 phosphorylation in a time-dependent manner. Rat cortical neuron cells were exposed to E2 (10 n
m
) at specific time intervals (0, 5, 10, 20, 30, 45, and 60 min), and cell lysates were immunoblotted for detection of pAkt or pERK1/2. A, Western Blot analysis showed that E2-induced Akt phosphorylation was first apparent within 5 min of exposure, was maximally induced at 10 min, diminished at 20 min, and reached maximal induction again at 30 min in a temporal pattern, consistent with ER–p85 coupling with significant and maximal efficacy at 10 and 30 min. B, pERK1/2 was linearly and significantly induced at 5 min, continued to rise 10 and 20 min, reached maximal activation at 30 min, and returned to baseline at 45 min. As a loading control, membranes were reblotted for total Akt and ERK1/2. Densitometry of phosphorylated forms were normalized to either total Akt or total ERK1/2, respectively. Expression of total proteins did not change across conditions or time. Data shown are from a single experiment and are representative of three independent experiments. *p < 0.05 versus control neurons; **p < 0.01 versus control neurons. Ctrl, Control.
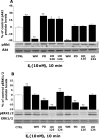
Figure 3.
E2-induced Akt and ERK1/2 phosphorylation at 10 min was blocked by inhibition of PI3K. Rat cortical neurons were treated with a phosphoinositide PI3K inhibitor [wortmannin (WM)], one of two different MAPK inhibitors [PD 98059 (PD) and UO126], or the negative control (UO124), alone or in combination with E2 (10 n
m
) for 10 min. A, Western blot analysis indicated that inhibition of PI3K with wortmannin completely blocked E2 activation of pAkt, whereas inhibitors of the MAPK pathway were without effect. B, Inhibition of PI3K with wortmannin (30 n
m
) completely blocked E2 activation of pERK1/2 at 10 min. Both of the MAP kinase kinase (MEK) inhibitors PD 98059 (20 μ
m
) and UO126 (20 μ
m
) completely blocked E2 induction of pERK1/2. The negative control UO124 (20 μ
m
) was without effect. No change was detected in either total AKT or ERK1/2. Data shown are from a single experiment and are representative of three independent experiments. *p < 0.05 versus control neurons; **p < 0.01 versus control neurons. CTRL, Control.
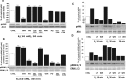
Figure 4.
Inhibition of PI3K at 30 min blocked E2 activation of pAkt but not pERK1/2. Rat cortical neurons were treated with either a phosphoinositide PI3K inhibitor [30 n
m
wortmannin (WM)], one of two different MAPK inhibitors [20 μ
m
PD 98059 (PD) and 20 μ
m
UO126], or the negative control [20 μ
m
UO124], alone or in combination with E2 (10 n
m
) for 30 min. A, E2 induced a significant increase in pAkt that was blocked by wortmannin at 30 min. As expected, MAPK inhibitors were without effect. B, E2 induced a significant increase in pERK1/2 at 30 min that was impervious to the PI3K inhibitor wortmannin but that showed pharmacological specificity because both MAPK inhibitors PD 98059 and UO124 completely blocked E2-induced pERK1/2. The inactive compound UO126 was with out effect. As a loading control, membranes were immunoblotted for either total Akt or ERK1/2, and the densitometry of phosphorylated forms were normalized to the respective total protein. Expression of total proteins did not change across conditions or time. Data shown are from a single experiment and are representative of three independent experiments. C, To ensure that loss of inhibitory control over E2-induced pERK was not a loss in pharmacological specificity, cortical neurons were exposed to an additional PI3K inhibitor, LY294004 (20 μ
m
) (LY). Cortical neurons were treated with PI3K inhibitors wortmannin (30 n
m
) or LY294004 (20 μ
m
), alone or in combination with E2 (10 n
m
) for 10 and 30 min. Western blot analysis indicated that wortmannin and LY294004 completely blocked E2 activation of pAkt at 10 and 30 min. D, Cortical neurons were treated under the same pharmacological conditions as in C. Wortmannin (30 n
m
) and LY294004 (20 μ
m
) completely blocked E2 activation of pERK1/2 at 10 min as found previously and were without effect at 30 min. Thus, the loss of inhibitory control over PI3K regulation of E2-inducible pERK at 30 min is not a shift in pharmacology but is indicative of a PI3K-independent pathway. Data shown are from a single experiment and are representative of two independent experiments. CTRL, Control. **p < 0.01.
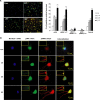
Figure 5.
E2-induced Akt and ERK1/2 phosphorylation occurs within the same cortical neurons in a time-dependent manner. Rat cortical neurons were exposed to E2 (10 n
m
) at different time points (0, 10, 20, and 30 min) and immunofluorescently labeled for expression of pAkt (green), pERK1/2 (red), or their coexpression (pAkt–pERK1/2 colocalization in yellow). A, Fluorescent microscopic images show relative density of pAkt- and pERK1/2-positive cells in the E2-treated group versus time 0 min. For each condition, microscopic fields were randomly selected, and >2000 neurons across three separate experiments were analyzed for pAkt and/or pERK1/2 immunoreactivity. E2-induced pAkt and pERK1/2 immunofluorescence was apparent at 10 min and maximal at 30 min, consistent with results of Western blot analyses. Quantitative analyses, shown in bar graph, indicate that the number of E2-induced pAkt-positive neurons occurred at 10 min, followed by a slight but not significant diminution at 20 min and subsequent dramatic rise to the maximal number of pAkt-positive neurons of nearly 70%. These data are consistent with those obtained with Western blot analyses. A significant increase in the number of E2-induced pERK1/2-positive neurons occurred at 10 min, followed by a slight rise at 20 min and subsequent dramatic rise to the maximal number of pERK1/2-positive neurons of 65%. These data are also consistent with those obtained with Western blot analyses. The number of cortical neurons exhibiting colocalization of E2-inducible pAkt and pERK1/2 was significantly increased above control at 10 min, rose slightly at 20 min, and exhibited the largest gain between 20 and 30 min to >60% of cortical neurons exhibiting coexpression of both pAkt and pERK1/2. Data shown are from a single experiment and are representative of three independent experiments. *p < 0.05 versus control neurons; **p < 0.01 versus control neurons. B, Immunofluorescent localization of E2-induced pAkt and pERK1/2. Rat cortical neurons were exposed to E2 (10 n
m
) at different time points (0, 10, 20, and 30 min) and immunofluorescently labeled for expression of pAkt (green), pERK1/2 (red), or their coexpression (pAkt–pERK1/2 colocalization in yellow) and DAPI nuclear counterstain (blue). Insets are higher-magnification images to highlight pAkt and pERK1/2 localization in neuronal processes. E2 activation-induced pAkt and pERK1/2 was apparent within both the cell body and neuronal processes at 10 min of exposure to E2. At 20 min of E2 exposure, pAkt remained apparent within the cell body but was markedly diminished in the processes. In contrast, pERK remained apparent within both the cell body and the processes across the observed time points. At 30 min, pAKT immunofluorescence returned to the processes and pERK immunofluorescence was sustained. Nuclear translocation of both pAkt and pERK was apparent at 10 min, appeared to be reduced at 20 min, and reemerged at 30 min. Data shown are from a single experiment and are representative of immunofluorescent analyses derived from three independent experiments.
Similar articles
- Extranuclear estrogen receptors mediate the neuroprotective effects of estrogen in the rat hippocampus.
Yang LC, Zhang QG, Zhou CF, Yang F, Zhang YD, Wang RM, Brann DW. Yang LC, et al. PLoS One. 2010 May 7;5(5):e9851. doi: 10.1371/journal.pone.0009851. PLoS One. 2010. PMID: 20479872 Free PMC article. - Nongenomic antiapoptotic signal transduction by estrogen in cultured cortical neurons.
Honda K, Shimohama S, Sawada H, Kihara T, Nakamizo T, Shibasaki H, Akaike A. Honda K, et al. J Neurosci Res. 2001 Jun 1;64(5):466-75. doi: 10.1002/jnr.1098. J Neurosci Res. 2001. PMID: 11391701 - Novel non-transcriptional mechanisms for estrogen receptor signaling in the cardiovascular system. Interaction of estrogen receptor alpha with phosphatidylinositol 3-OH kinase.
Simoncini T, Fornari L, Mannella P, Varone G, Caruso A, Liao JK, Genazzani AR. Simoncini T, et al. Steroids. 2002 Nov;67(12):935-9. doi: 10.1016/s0039-128x(02)00040-5. Steroids. 2002. PMID: 12398989 Review. - Interactions of estrogens and insulin-like growth factor-I in the brain: implications for neuroprotection.
Cardona-Gómez GP, Mendez P, DonCarlos LL, Azcoitia I, Garcia-Segura LM. Cardona-Gómez GP, et al. Brain Res Brain Res Rev. 2001 Nov;37(1-3):320-34. doi: 10.1016/s0165-0173(01)00137-0. Brain Res Brain Res Rev. 2001. PMID: 11744097 Review.
Cited by
- The Role of PIK3R1 in Metabolic Function and Insulin Sensitivity.
Tsay A, Wang JC. Tsay A, et al. Int J Mol Sci. 2023 Aug 11;24(16):12665. doi: 10.3390/ijms241612665. Int J Mol Sci. 2023. PMID: 37628845 Free PMC article. Review. - Network Pharmacology and Molecular Docking Reveal the Mechanism of Isodon ternifolius (D. Don) Kudo Against Liver Fibrosis.
Deng J, Qin L, Zhou Z. Deng J, et al. Drug Des Devel Ther. 2023 Aug 7;17:2335-2351. doi: 10.2147/DDDT.S412818. eCollection 2023. Drug Des Devel Ther. 2023. PMID: 37576085 Free PMC article. - Cardiometabolic health, menopausal estrogen therapy and the brain: How effects of estrogens diverge in healthy and unhealthy preclinical models of aging.
Daniel JM, Lindsey SH, Mostany R, Schrader LA, Zsombok A. Daniel JM, et al. Front Neuroendocrinol. 2023 Jul;70:101068. doi: 10.1016/j.yfrne.2023.101068. Epub 2023 Apr 13. Front Neuroendocrinol. 2023. PMID: 37061205 Free PMC article. Review. - Immune regulation based on sex differences in ischemic stroke pathology.
Niu P, Li L, Zhang Y, Su Z, Wang B, Liu H, Zhang S, Qiu S, Li Y. Niu P, et al. Front Immunol. 2023 Jan 30;14:1087815. doi: 10.3389/fimmu.2023.1087815. eCollection 2023. Front Immunol. 2023. PMID: 36793730 Free PMC article. Review. - Stress-altering anterior insular cortex activity affects risk decision-making behavior in mice of different sexes.
Shi T, Feng S, Zhou Z, Li F, Fu Y, Zhou W. Shi T, et al. Front Cell Neurosci. 2023 Jan 24;17:1094808. doi: 10.3389/fncel.2023.1094808. eCollection 2023. Front Cell Neurosci. 2023. PMID: 36761354 Free PMC article.
References
- Abbondanza C, de Falco A, Nigro V, Medici N, Armetta I, Molinari AM, Moncharmont B, Puca GA. Characterization and epitope mapping of a new panel of monoclonal antibodies to estradiol receptor. Steroids. 1993;58:4–12. - PubMed
- Alexaki VI, Charalampopoulos I, Kampa M, Vassalou H, Theodoropoulos P, Stathopoulos EN, Hatzoglou A, Gravanis A, Castanas E. Estrogen exerts neuroprotective effects via membrane estrogen receptors and rapid Akt/NOS activation. FASEB J. 2004;18:1594–1596. - PubMed
- Blair LA, Marshall J. IGF-1 modulates N and L calcium channels in a PI 3-kinase-dependent manner. Neuron. 1997;19:421–429. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous