High levels of Cre expression in neuronal progenitors cause defects in brain development leading to microencephaly and hydrocephaly - PubMed (original) (raw)
. 2006 Sep 13;26(37):9593-602.
doi: 10.1523/JNEUROSCI.2815-06.2006.
Claudio Scuoppo, Itaru Imayoshi, Riccardo Taulli, Walter Dastrù, Valentina Sala, Ulrich A K Betz, Patrizia Muzzi, Daniela Martinuzzi, Alessandro E Vercelli, Ryoichiro Kageyama, Carola Ponzetto
Affiliations
- PMID: 16971543
- PMCID: PMC6674592
- DOI: 10.1523/JNEUROSCI.2815-06.2006
High levels of Cre expression in neuronal progenitors cause defects in brain development leading to microencephaly and hydrocephaly
Paolo E Forni et al. J Neurosci. 2006.
Abstract
Hydrocephalus is a common and variegated pathology often emerging in newborn children after genotoxic insults during pregnancy (Hicks and D'Amato, 1980). Cre recombinase is known to have possible toxic effects that can compromise normal cell cycle and survival. Here we show, by using three independent nestin Cre transgenic lines, that high levels of Cre recombinase expression into the nucleus of neuronal progenitors can compromise normal brain development. The transgenics analyzed are the nestin Cre Balancer (Bal1) line, expressing the Cre recombinase with a nuclear localization signal, and two nestin CreER(T2) (Cre recombinase fused with a truncated estrogen receptor) mice lines with different levels of expression of a hybrid CreER(T2) recombinase that translocates into the nucleus after tamoxifen treatment. All homozygous Bal1 nestin Cre embryos displayed reduced neuronal proliferation, increased aneuploidy and cell death, as well as defects in ependymal lining and lamination of the cortex, leading to microencephaly and to a form of communicating hydrocephalus. An essentially overlapping phenotype was observed in the two nestin CreER(T2) transgenic lines after tamoxifen mediated-CreER(T2) translocation into the nucleus. Neither tamoxifen-treated wild-type nor nestin CreER(T2) oil-treated control mice displayed these defects. These results indicate that some forms of hydrocephalus may derive from a defect in neuronal precursors proliferation. Furthermore, they underscore the potential risks for developmental studies of high levels of nuclear Cre in neurogenic cells.
Figures
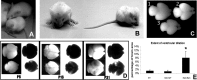
Figure 1.
Bal1 nestin Cre homozygous mice (at P14 in the picture) are smaller than their wild-type littermates (A), have an enlarged head, and display postural defects (B). C, Whole brains of E19.5 wild-type (WT) (1), Bal1 nestin Cre heterozygous (2), and Bal1 nestin Cre homozygous (3) embryos. D, Brains of Bal1 nestin Cre homozygous mice at the indicated postnatal age (bottom row) are significantly smaller with respect to those of control wild-type littermates (top row) and display progressive ventricular enlargement. Brains are shown in normal light (dark field) and transilluminated (bright field). E, The extent of ventricular dilation at E19.5 is expressed as percentage of brain area occupied by the ventricles. Measurements were done as indicated in Materials and Methods. Error bars represent mean ± SD; n = 5 for each genotype. *p < 0.001, t test.
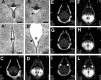
Figure 2.
A, B, Cresyl violet staining of coronal sections trough the superior colliculi from wild-type (A) and homozygous (B) Bal1 nestin Cre mice at P6. In homozygous Bal1 nestin Cre mice, the Sylvius aqueduct is pervious but displays defects in ependymal lining (arrowhead; B). Cresyl violet staining of coronal brain sections from wild-type (A′) and homozygous (B′) Bal1 nestin Cre mice at P6 show defects in ependymal lining (arrowhead) and in the third ventricle (III). MRI analysis (horizontal sections) of wild-type (C, E, G, I) and homozygous (D, F, H, L) Bal1 nestin Cre littermates at P6 shows enlargement of the lateral (LV), third (III), and fourth (IV) ventricles. Although there are no obvious signs of obstruction, the Sylvius aqueduct (SA; F) appears dilated. Scale bars: A, B, 50 μm; A′, B′, 100 μm.
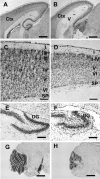
Figure 3.
Cresyl violet staining of brain sagittal sections from wild-type (A, C, E, G) and homozygous (B, D, F, H) Bal1 nestin Cre mice at P8. A, B, The thickness of the parieto-occipital cerebral cortex (ctx) is reduced in homozygous Bal1 nestin Cre mice. C, D, Higher magnification shows reduced cellularity and reduced organization of the cortical layers (I–VI) in the occipital cortex. SP, Subplate. The dentate gyrus (DG) of the hippocampus is reduced and less organized (E, F) and the cerebellum is underdeveloped (G, H) in homozygous Bal1 nestin Cre mice. Scale bars: A, B, G, H, 500 μm; C–F, 100 μm.
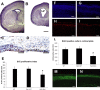
Figure 4.
A, B, Cresyl violet staining of brain coronal sections from wild-type (A) and homozygous (B) Bal1 nestin Cre littermates at P2. LV, Lateral ventricles. C, D, Ki-67 staining at E19.5 shows reduction of positive cells in the SVZ of homozygous Bal1 nestin Cre mice (D) compared with wild-type littermates (C). E, Quantification of Ki-67-positive cells in the SVZ of homozygous Bal1 and heterozygous and wild-type controls (WT) (see Material and Methods; n = 3). F–I, BrdU staining of the cortex of P2 newborn mice analyzed 4.5 d after injection of pregnant mothers. BrdU-positive cells in the cortex of Bal1 nestin Cre homozygotes (I) are significantly reduced compared with wild-type controls (H). F, G, DAPI staining. L, Quantification of BrdU-positive cells in the cortical plate of P2 animals of the same litter 4.5 d after injection. M, N, Cre immunostaining of the pial layers of the cortex in P2 homozygous Bal1 nestin Cre mice (N) and heterozygous littermates (M) reveals a reduction of Cre-positive progenitors that have reached the cortex. E–L, n = 3 for each genotype; values are mean ± SD. *p < 0.001, t test. Scale bars: A, B, 500 μm; C, D, 50 μm; F, G, H, I, M, N, 100 μm.
Figure 5.
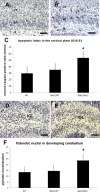
Immunostaining for cleaved caspase-3 on E19.5 wild-type (A) and homozygous (B) Bal1 nestin Cre brain sections reveals an increased number of cells undergoing apoptosis in the intermediate zone (IZ), throughout the pre-plate (PP), and in the cortical plate (CP). C, Quantification of cleaved caspase-3 in developing cortex (E19.5). Immunostaining for cleaved caspase-3 on sections of P3 wild-type (WT; D) and homozygous (E) Bal1 nestin Cre cerebellum sections shows an increased number of cleaved caspase-3-positive cells in the inner granule layer (IGL) of homozygous Bal1 nestin Cre mice (E). F, Quantification of pyknotic nuclei in cerebellum after cresyl violet staining on P2 pups of the same litter. n = 3 for each genotype; values are mean ± SD.*p < 0.001, t test (C, F). Scale bars, 50 μm.
Figure 6.
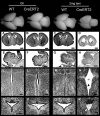
Top row, Wild-type and heterozygous CreERT2 line 1 whole brains at E19.5 after the indicated treatments. Cresyl violet staining of coronal brain sections (A–D), sagittal cerebellar sections (E–H), coronal sections of the third ventricle (III) (I–N), and coronal sections of the Sylvius aqueduct (O–R). Heterozygous nestin CreERT2 embryos treated with vehicle alone (oil) display normal brain (B) and normal cerebellar development (F) and are thus indistinguishable from wild-type (WT) controls treated with either oil (A, E) or 2 mg of tamoxifen (C, G; tam). Tamoxifen-treated heterozygous nestin CreERT2 display severe signs of microencephaly and ventricular dilation (D), reduced development of the cerebellum (H), ventricular dilation (D, N, R), and defects in ependymal lining of the third ventricle (III) and Sylvius aqueduct (N, R, arrows). Scale bars: A–D, 500 μm; E–H, 200 μm; I–R,100 μm.
Figure 7.
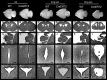
Top row, Wild-type and heterozygous CreERT2 line 4 whole brains at E19.5 after the indicated treatments. Cresyl violet staining of coronal brain sections (A–F), sagittal cerebellar sections (G–L), coronal sections of the third ventricle (III) (M–R), and Sylvius aqueduct (S–X). Heterozygous nestin CreERT2 embryos treated with vehicle alone (oil) (B, H, N, T) or with 2 mg of tamoxifen (tam) (D, J, P, V) are indistinguishable from oil-treated (A, G, M, S) and 2 mg tamoxifen-treated (C, I, O, U) wild-type (WT) animals. Tamoxifen-treated (8 mg) wild-type embryos are indistinguishable from the previous controls (E, K, Q, W), whereas 8 mg tamoxifen-treated heterozygous nestin CreERT2 (F, L, R, X) display severe signs of microencephaly and ventricular dilation (F), reduced development of the cerebellum (L), defects in ependymal lining in the third ventricle (R) and defects in Sylvius aqueduct ependymal lining (X) (arrowheads). Scale bars: A–F, 500 μm; G–L, 200 μm; M–X, 100 μm.
Figure 8.
Ki-67 labeling (dark stain) at E19.5 on coronal sections from oil-treated (A) and 2 mg tamoxifen-treated (TAM) (B) nestin CreERT2 line 1 heterozygous embryos reveals a reduction of the number of proliferating cells in the SVZ and in the intermediate zone (IZ) of tamoxifen-treated embryos (B) with respect to oil-treated control (A). LV, Lateral ventricle. C, Quantification of Ki-67-positive cells in the SVZ at E19.5 in heterozygous CreERT2 line 1 and line 4 and wild-type controls embryos after the indicated treatment performed at E10.5. Error bars represent mean ± SD; n = 3 for each genotype. *p < 0.001, t test.
Similar articles
- Specificity and efficiency of reporter expression in adult neural progenitors vary substantially among nestin-CreER(T2) lines.
Sun MY, Yetman MJ, Lee TC, Chen Y, Jankowsky JL. Sun MY, et al. J Comp Neurol. 2014 Apr 1;522(5):1191-208. doi: 10.1002/cne.23497. J Comp Neurol. 2014. PMID: 24519019 Free PMC article. - Inducible site-specific recombination in neural stem/progenitor cells.
Chen J, Kwon CH, Lin L, Li Y, Parada LF. Chen J, et al. Genesis. 2009 Feb;47(2):122-31. doi: 10.1002/dvg.20465. Genesis. 2009. PMID: 19117051 Free PMC article. - Nestin-CreER mice reveal DNA synthesis by nonapoptotic neurons following cerebral ischemia hypoxia.
Burns KA, Ayoub AE, Breunig JJ, Adhami F, Weng WL, Colbert MC, Rakic P, Kuan CY. Burns KA, et al. Cereb Cortex. 2007 Nov;17(11):2585-92. doi: 10.1093/cercor/bhl164. Epub 2007 Jan 27. Cereb Cortex. 2007. PMID: 17259645 - Endothelial-Specific Cre Mouse Models.
Payne S, De Val S, Neal A. Payne S, et al. Arterioscler Thromb Vasc Biol. 2018 Nov;38(11):2550-2561. doi: 10.1161/ATVBAHA.118.309669. Arterioscler Thromb Vasc Biol. 2018. PMID: 30354251 Free PMC article. Review. - Development of mice with brain-specific deletion of floxed glud1 (glutamate dehydrogenase 1) using cre recombinase driven by the nestin promoter.
Karaca M, Maechler P. Karaca M, et al. Neurochem Res. 2014;39(3):456-9. doi: 10.1007/s11064-013-1041-0. Epub 2013 Apr 18. Neurochem Res. 2014. PMID: 23595828 Review.
Cited by
- A role for presenilins in autophagy revisited: normal acidification of lysosomes in cells lacking PSEN1 and PSEN2.
Zhang X, Garbett K, Veeraraghavalu K, Wilburn B, Gilmore R, Mirnics K, Sisodia SS. Zhang X, et al. J Neurosci. 2012 Jun 20;32(25):8633-48. doi: 10.1523/JNEUROSCI.0556-12.2012. J Neurosci. 2012. PMID: 22723704 Free PMC article. - RNA Binding Motif 5 Gene Deletion Modulates Cell Signaling in a Sex-Dependent Manner but Not Hippocampal Cell Death.
Farooq J, Snyder K, Janesko-Feldman K, Gorse K, Vagni VA, Kochanek PM, Jackson TC. Farooq J, et al. J Neurotrauma. 2022 Apr;39(7-8):577-589. doi: 10.1089/neu.2021.0362. Epub 2022 Mar 1. J Neurotrauma. 2022. PMID: 35152732 Free PMC article. - Conditional deletion of pejvakin in adult outer hair cells causes progressive hearing loss in mice.
Harris SL, Kazmierczak M, Pangršič T, Shah P, Chuchvara N, Barrantes-Freer A, Moser T, Schwander M. Harris SL, et al. Neuroscience. 2017 Mar 6;344:380-393. doi: 10.1016/j.neuroscience.2016.12.055. Epub 2017 Jan 9. Neuroscience. 2017. PMID: 28089576 Free PMC article. - A Study on Potential Sources of Perineuronal Net-Associated Sema3A in Cerebellar Nuclei Reveals Toxicity of Non-Invasive AAV-Mediated Cre Expression in the Central Nervous System.
Gimenez GA, Romijn M, van den Herik J, Meijer W, Eggers R, Hobo B, De Zeeuw CI, Canto CB, Verhaagen J, Carulli D. Gimenez GA, et al. Int J Mol Sci. 2025 Jan 19;26(2):819. doi: 10.3390/ijms26020819. Int J Mol Sci. 2025. PMID: 39859534 Free PMC article.
References
- Alonzi T, Middleton G, Wyatt S, Buchman V, Betz UA, Muller W, Musiani P, Poli V, Davies AM. Role of STAT3 and PI 3-kinase/Akt in mediating the survival actions of cytokines on sensory neurons. Mol Cell Neurosci. 2001;18:270–282. - PubMed
- Aolad HM, Inouye M, Hayasaka S, Darmanto W, Murata Y. Congenital hydrocephalus caused by exposure to low level X-radiation at early gestational stage in mice. Biol Sci Space. 1998;12:256–257. - PubMed
- Assadi AH, Zhang G, Beffert U, McNeil RS, Renfro AL, Niu S, Quattrocchi CC, Antalffy BA, Sheldon M, Armstrong DD, Wynshaw-Boris A, Herz J, D’Arcangelo G, Clark GD. Interaction of reelin signaling and Lis1 in brain development. Nat Genet. 2003;35:270–276. - PubMed
- Baba Y, Nakano M, Yamada Y, Saito I, Kanegae Y. Practical range of effective dose for Cre recombinase-expressing recombinant adenovirus without cell toxicity in mammalian cells. Microbiol Immunol. 2005;49:559–570. - PubMed
- Bearer CF. L1 cell adhesion molecule signal cascades: targets for ethanol developmental neurotoxicity. Neurotoxicology. 2001;22:625–633. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases