Pathogenesis of West Nile Virus infection: a balance between virulence, innate and adaptive immunity, and viral evasion - PubMed (original) (raw)
Review
Pathogenesis of West Nile Virus infection: a balance between virulence, innate and adaptive immunity, and viral evasion
Melanie A Samuel et al. J Virol. 2006 Oct.
No abstract available
Figures
FIG. 1.
E16 contact residues and binding of WNV virions. (A) E16 contact residues (red) on DIII of the WNV envelope protein are located in the amino terminus (residues 302 to 309) and three strand-connecting loops, BC (residues 330 to 333), DE (residues 365 to 368), and FG (residues 389 to 391). (B) Pseudoatomic model of the cryoelectron microscopic reconstruction of the WNV virion. The E16 structural epitope is mapped in magenta. (C) Saturation binding of E16 on the WNV particle. E16 is predicted to bind 120 out of 180 potential epitopes with exclusion from the inner fivefold axis. (D and E) Magnified regions of the boxed areas in panel C. This figure is reprinted with permission from Macmillan Publishers Ltd. (Nature **437:**764-768, copyright 2005).
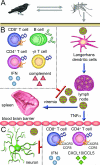
FIG. 2.
WNV dissemination and immune system control. (A) WNV is maintained in nature in an enzootic mosquito-bird-mosquito transmission cycle. (B) Following Culex mosquito inoculation, WNV replicates in skin Langerhans dendritic cells, which traffic the virus to the lymph node, where further replication ensues. Following induction of a primary viremia, WNV spreads to other peripheral organs. Several aspects of the innate and adaptive immune response limit WNV replication in the periphery. IFN-α/β acts as an antiviral agent that restricts viral translation and replication soon after infection. B cells and antibody (primarily IgM) modulate viral levels in serum and prevent early CNS seeding, while complement is required for efficient priming of humoral and cellular immune responses. IFN-γ-secreting γδ T cells control viral replication through direct antiviral mechanisms and contribute to the generation of adaptive immune responses. CD4+ and CD8+ T cells participate in viral clearance from peripheral tissues. (C) Following replication in the periphery, WNV spreads to the CNS possibly through TNF-α-mediated changes in BBB permeability. Neurons are the primary target of WNV in the brain and spinal cord. IFN-α/β is required to control WNV infection in the CNS and may prolong neuronal survival. The chemokines CXCL10 and CCL5 and their cognate ligands CXCR3 and CCR5 aid in recruiting CD4+ and CD8+ T cells and monocytes to the CNS, where they function to clear virus from infected tissues.
Similar articles
- Genetic diversity in the collaborative cross model recapitulates human West Nile virus disease outcomes.
Graham JB, Thomas S, Swarts J, McMillan AA, Ferris MT, Suthar MS, Treuting PM, Ireton R, Gale M Jr, Lund JM. Graham JB, et al. mBio. 2015 May 5;6(3):e00493-15. doi: 10.1128/mBio.00493-15. mBio. 2015. PMID: 25944860 Free PMC article. - Immunological headgear: antiviral immune responses protect against neuroinvasive West Nile virus.
Klein RS, Diamond MS. Klein RS, et al. Trends Mol Med. 2008 Jul;14(7):286-94. doi: 10.1016/j.molmed.2008.05.004. Epub 2008 Jun 6. Trends Mol Med. 2008. PMID: 18539532 Review. - The neuroimmune response to West Nile virus.
Fredericksen BL. Fredericksen BL. J Neurovirol. 2014 Apr;20(2):113-21. doi: 10.1007/s13365-013-0180-z. Epub 2013 Jul 11. J Neurovirol. 2014. PMID: 23843081 Free PMC article. Review. - West Nile virus: immunity and pathogenesis.
Lim SM, Koraka P, Osterhaus AD, Martina BE. Lim SM, et al. Viruses. 2011 Jun;3(6):811-28. doi: 10.3390/v3060811. Epub 2011 Jun 15. Viruses. 2011. PMID: 21994755 Free PMC article. Review. - STING is required for host defense against neuropathological West Nile virus infection.
McGuckin Wuertz K, Treuting PM, Hemann EA, Esser-Nobis K, Snyder AG, Graham JB, Daniels BP, Wilkins C, Snyder JM, Voss KM, Oberst A, Lund J, Gale M Jr. McGuckin Wuertz K, et al. PLoS Pathog. 2019 Aug 15;15(8):e1007899. doi: 10.1371/journal.ppat.1007899. eCollection 2019 Aug. PLoS Pathog. 2019. PMID: 31415679 Free PMC article.
Cited by
- A Japanese encephalitis virus genotype 5 molecular clone is highly neuropathogenic in a mouse model: impact of the structural protein region on virulence.
de Wispelaere M, Frenkiel MP, Desprès P. de Wispelaere M, et al. J Virol. 2015 Jun;89(11):5862-75. doi: 10.1128/JVI.00358-15. Epub 2015 Mar 18. J Virol. 2015. PMID: 25787283 Free PMC article. - Axonal transport mediates West Nile virus entry into the central nervous system and induces acute flaccid paralysis.
Samuel MA, Wang H, Siddharthan V, Morrey JD, Diamond MS. Samuel MA, et al. Proc Natl Acad Sci U S A. 2007 Oct 23;104(43):17140-5. doi: 10.1073/pnas.0705837104. Epub 2007 Oct 15. Proc Natl Acad Sci U S A. 2007. PMID: 17939996 Free PMC article. - HTS-driven discovery of new chemotypes with West Nile Virus inhibitory activity.
Chung DH, Jonsson CB, Maddox C, McKellip SN, Moore BP, Heil M, White EL, Ananthan S, Li Q, Feng S, Rasmussen L. Chung DH, et al. Molecules. 2010 Mar 12;15(3):1690-704. doi: 10.3390/molecules15031690. Molecules. 2010. PMID: 20336008 Free PMC article. - Interferon regulatory factor-1 (IRF-1) shapes both innate and CD8(+) T cell immune responses against West Nile virus infection.
Brien JD, Daffis S, Lazear HM, Cho H, Suthar MS, Gale M Jr, Diamond MS. Brien JD, et al. PLoS Pathog. 2011 Sep;7(9):e1002230. doi: 10.1371/journal.ppat.1002230. Epub 2011 Sep 1. PLoS Pathog. 2011. PMID: 21909274 Free PMC article. - Matrix metalloproteinase 9 facilitates West Nile virus entry into the brain.
Wang P, Dai J, Bai F, Kong KF, Wong SJ, Montgomery RR, Madri JA, Fikrig E. Wang P, et al. J Virol. 2008 Sep;82(18):8978-85. doi: 10.1128/JVI.00314-08. Epub 2008 Jul 16. J Virol. 2008. PMID: 18632868 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
- AI53870/AI/NIAID NIH HHS/United States
- AI61373/AI/NIAID NIH HHS/United States
- U01 AI053870/AI/NIAID NIH HHS/United States
- U01 AI061373/AI/NIAID NIH HHS/United States
- U54 AI057160/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical