Functional significance of the rapid regulation of brain estrogen action: where do the estrogens come from? - PubMed (original) (raw)
Review
Functional significance of the rapid regulation of brain estrogen action: where do the estrogens come from?
Charlotte A Cornil et al. Brain Res. 2006.
Abstract
Estrogens exert a wide variety of actions on reproductive and non-reproductive functions. These effects are mediated by slow and long lasting genomic as well as rapid and transient non-genomic mechanisms. Besides the host of studies demonstrating the role of genomic actions at the physiological and behavioral level, mounting evidence highlights the functional significance of non-genomic effects. However, the source of the rapid changes in estrogen availability that are necessary to sustain their fast actions is rarely questioned. For example, the rise of plasma estrogens at pro-estrus that represents one of the fastest documented changes in plasma estrogen concentration appears too slow to explain these actions. Alternatively, estrogen can be synthesized in the brain by the enzyme aromatase providing a source of locally high concentrations of the steroid. Furthermore, recent studies demonstrate that brain aromatase can be rapidly modulated by afferent inputs, including glutamatergic afferents. A role for rapid changes in estrogen production in the central nervous system is supported by experiments showing that acute aromatase inhibition affects nociception as well as male sexual behavior and that preoptic aromatase activity is rapidly (within min) modulated following mating. Such mechanisms thus fulfill the gap existing between the fast actions of estrogen and their mode of production and open new avenues for the understanding of estrogenic effects on the brain.
Figures
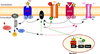
Figure 1. Schematic overview of some cellular pathways affected by estradiol
In order to simplify this drawing only selected intracellular events are presented. Upon binding of their ligand (L), G-protein coupled receptors (GPCR, red receptor) such as metabotropic glutamate receptors (mGluR), 5-HT receptors, GABAB receptors, µ opioids receptors, α- and β-adrenergic receptors activate different intracellular signaling pathways through their G protein (made of α and β subunits). The activation of the G protein can lead to the activation of phospholipase C (PLC; not shown) that catalyzes the hydrolysis of membrane-associated phosphatidyl-inositol 4,5-biphosphate (PIP2) into 1,4,5 triphosphate (IP3) and diacylglycerol (DAG). IP3 induces calcium release from the intracellular stores (reticulum endoplasmic). DAG activates protein kinase C (PKC). In turn, PKC can stimulate, through phosphorylation (P), the activity of adenylate cyclase (AC) to produce cAMP and activate protein kinase A (PKA). PKA can also phosphorylate various proteins such as other receptors, ion channels (such as Type-L voltage-gated Ca2+ channels [L-VGCC], G-protein-coupled, inwardly rectifying K+ channels [GIRK] or the small conductance, Ca2+-dependent K+ channel [SK]) or cAMP-responsive binding protein (CREB). Rapid non genomic signaling: 1/ Estradiol (E2, yellow star) can allosterically modulate the activity of ionotropic receptors (such as acetylcholine, kainate or NMDA receptors, orange receptors) or GPCR by directly interacting with this receptor. 2/ E2 can also activate a membrane-bound estrogen receptors (mER, blue receptor; the term is used here in a general way to cover both ERα and ERβ associated with the membrane and the more recently identified estrogen membrane receptor [GPR30, ER-X, etc] specifically named mER) that is coupled to a G protein. Thereby, E2 can modulate the activity of ionic conductance through phosphorylation of ionotropic receptors or uncoupling (dashed arrows) of GPCR from their ionic channels or intracellular effectors (not shown). It can also mobilize intracellular Ca2+ through activation of PLC or uncoupling of a GPCR from PLC (not shown). Delayed genomic signaling: E2 can bind to nuclear estrogen receptors (ER) that form dimers and bind the estrogen-responsive element (ERE) on the DNA resulting in the activation of the transcription of specific genes. In addition, rapid effects of E2 mediated through mER resulting in the activation of protein kinases can lead to phosphorylation of CREB, which can alter gene transcription through its interaction with the cAMP responsive element (CRE; indirect genomic effect).
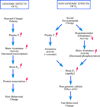
Figure 2. Potential sources of estrogens in males associated with genomic and non-genomic effects on physiology and behavior
On the one hand, genomic effects are associated with a slow rise of testosterone concentrations in the plasma. These changes are correlated with changes in testis function at puberty and across seasons. This rise in plasma testosterone results in an increased aromatase activity in the brain (through an activation of transcription of its gene). The estrogens produced locally by the aromatization of testosterone influence the transcription of various genes ultimately resulting in long lasting behavioral changes. On the other hand, non-genomic effects are associated with rapid changes in estrogens bio-availability. Such rapid changes in brain estrogen concentrations could result from variations of plasma testosterone that is subsequently aromatized locally into estrogen by brain aromatase as well as from variations in neurotransmitters’ activity, such as glutamate or dopamine, that rapidly regulate brain aromatase activity and the subsequent production of estrogen. These fast changes in brain estrogen concentrations could in turn induce fast non-genomic actions in specific cell populations that would ultimately result in fast behavioral effects. In both cases, aromatase concentration remains constant and either the substrate of the enzyme (testosterone) rapidly becomes more concentrated resulting in an increased product formation or the substrate remains constant but the rate of conversion into estrogen (the enzymatic efficiency) is rapidly increased.
Similar articles
- Effects of testosterone and its metabolites on aromatase-immunoreactive cells in the quail brain: relationship with the activation of male reproductive behavior.
Balthazart J, Foidart A, Absil P, Harada N. Balthazart J, et al. J Steroid Biochem Mol Biol. 1996 Jan;56(1-6 Spec No):185-200. doi: 10.1016/0960-0760(95)00236-7. J Steroid Biochem Mol Biol. 1996. PMID: 8603040 Review. - Preoptic aromatase modulates male sexual behavior: slow and fast mechanisms of action.
Balthazart J, Baillien M, Cornil CA, Ball GF. Balthazart J, et al. Physiol Behav. 2004 Nov 15;83(2):247-70. doi: 10.1016/j.physbeh.2004.08.025. Physiol Behav. 2004. PMID: 15488543 Review. - Rapid changes in production and behavioral action of estrogens.
Balthazart J, Cornil CA, Taziaux M, Charlier TD, Baillien M, Ball GF. Balthazart J, et al. Neuroscience. 2006;138(3):783-91. doi: 10.1016/j.neuroscience.2005.06.016. Epub 2005 Dec 15. Neuroscience. 2006. PMID: 16359807 Review. - Diversity of mechanisms involved in aromatase regulation and estrogen action in the brain.
Charlier TD, Cornil CA, Ball GF, Balthazart J. Charlier TD, et al. Biochim Biophys Acta. 2010 Oct;1800(10):1094-105. doi: 10.1016/j.bbagen.2009.12.010. Epub 2010 Jan 12. Biochim Biophys Acta. 2010. PMID: 20060879 Free PMC article. Review. - Glutamate released in the preoptic area during sexual behavior controls local estrogen synthesis in male quail.
de Bournonville C, Smolders I, Van Eeckhaut A, Ball GF, Balthazart J, Cornil CA. de Bournonville C, et al. Psychoneuroendocrinology. 2017 May;79:49-58. doi: 10.1016/j.psyneuen.2017.02.002. Epub 2017 Feb 8. Psychoneuroendocrinology. 2017. PMID: 28259043 Free PMC article.
Cited by
- Sex differences in brain aromatase activity: genomic and non-genomic controls.
Balthazart J, Charlier TD, Cornil CA, Dickens MJ, Harada N, Konkle AT, Voigt C, Ball GF. Balthazart J, et al. Front Endocrinol (Lausanne). 2011 Sep 29;2:34. doi: 10.3389/fendo.2011.00034. eCollection 2011. Front Endocrinol (Lausanne). 2011. PMID: 22645508 Free PMC article. - Female rats are more vulnerable to the long-term consequences of neonatal inflammatory injury.
LaPrairie JL, Murphy AZ. LaPrairie JL, et al. Pain. 2007 Nov;132 Suppl 1(Suppl 1):S124-S133. doi: 10.1016/j.pain.2007.08.010. Epub 2007 Sep 29. Pain. 2007. PMID: 17904745 Free PMC article. - Presynaptic control of rapid estrogen fluctuations in the songbird auditory forebrain.
Remage-Healey L, Dong S, Maidment NT, Schlinger BA. Remage-Healey L, et al. J Neurosci. 2011 Jul 6;31(27):10034-8. doi: 10.1523/JNEUROSCI.0566-11.2011. J Neurosci. 2011. PMID: 21734295 Free PMC article. - In vivo Hippocampal Serotonin Dynamics in Male and Female Mice: Determining Effects of Acute Escitalopram Using Fast Scan Cyclic Voltammetry.
Saylor RA, Hersey M, West A, Buchanan AM, Berger SN, Nijhout HF, Reed MC, Best J, Hashemi P. Saylor RA, et al. Front Neurosci. 2019 Apr 23;13:362. doi: 10.3389/fnins.2019.00362. eCollection 2019. Front Neurosci. 2019. PMID: 31110471 Free PMC article.
References
- Abdelgadir SE, Resko JA, Ojeda SR, Lephart ED, McPhaul MJ, Roselli CE. Androgens regulate aromatase cytochrome P450 messenger ribonucleic acid in rat brain. Endocrinology. 1994;135:395–401. - PubMed
- Abraham IM, Todman MG, Korach KS, Herbison AE. Critical in vivo roles for classical estrogen receptors in rapid estrogen actions on intracellular signalling in mouse brain. Endocrinology. 2004;145:3055–3061. - PubMed
- Absil P, Baillien M, Ball GF, Panzica GC, Balthazart J. The control of preoptic aromatase activity by afferent inputs in Japanese quail. Brain Res Rev. 2001;37:38–58. - PubMed
- Adkins EK, Boop JJ, Koutnik DL, Morris JB, Pnieswski EE. Further evidence that androgen aromatization is essential for the activation of in male quail. Physiology and behavior. 1980;24:441–446. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous