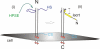Heparanase deglycanation of syndecan-1 is required for binding of the epithelial-restricted prosecretory mitogen lacritin - PubMed (original) (raw)
Heparanase deglycanation of syndecan-1 is required for binding of the epithelial-restricted prosecretory mitogen lacritin
Peisong Ma et al. J Cell Biol. 2006.
Erratum in
- J Cell Biol. 2011 Jan 24;192(2):365
Abstract
Cell surface heparan sulfate (HS) proteoglycans are carbohydrate-rich regulators of cell migratory, mitogenic, secretory, and inflammatory activity that bind and present soluble heparin-binding growth factors (e.g., fibroblast growth factor, Wnt, Hh, transforming growth factor beta, amphiregulin, and hepatocyte growth factor) to their respective signaling receptors. We demonstrate that the deglycanated core protein of syndecan-1 (SDC1) and not HS chains nor SDC2 or -4, appears to target the epithelial selective prosecretory mitogen lacritin. An important and novel step in this mechanism is that binding necessitates prior partial or complete removal of HS chains by endogenous heparanase. This limits lacritin activity to sites where heparanase appears to predominate, such as sites of exocrine cell migration, secretion, renewal, and inflammation. Binding is mutually specified by lacritin's C-terminal mitogenic domain and SDC1's N terminus. Heparanase modification of the latter transforms a widely expressed HS proteoglycan into a highly selective surface-binding protein. This novel example of cell specification through extracellular modification of an HS proteoglycan has broad implications in development, homeostasis, and disease.
Figures
Figure 1.
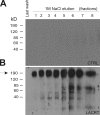
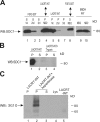
Lacritin affinity purification of cell surface SDC1. Detergent lysates of surface biotinylated HSG cells were incubated overnight in detergent and physiological NaCl with intein-chitin columns either lacking (A) or containing (B) lacritin. After extensive washing in the same buffer, the columns were eluted with 1 M NaCl, and eluted proteins were identified by blotting with streptavidin-peroxidase. A predominant 190-kD biotinylated protein eluting from the lacritin column was identified by mass spectrometry as human SDC1.
Figure 2.
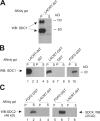
Lacritin binding to SDC1 is independent of complete HS/CS glycosaminoglycans. (A) Lacritin affinity precipitation of human SDC1 multimers stably expressed by HEK293T cells. Lacritin-intein beads were incubated with cell lysates, washed extensively, and treated with heparitinase I/chondroitinase ABC. Pellet (P) and supernatant (S) from the centrifuged digest were then blotted with mAb B-B4 for SDC1 core protein. (B) Lacritin-intein, lacritin-GST, FGF2-GST, intein, and GST beads were incubated with lysates from the same HEK293T cells stably expressing human SDC1. Precipitates were washed, treated, centrifuged, and blotted as in A. (C) Lacritin-intein and FGF2-GST beads were incubated with lysate of HEK293T cells stably expressing human SDC2 or lysate of another HEK293T cell line stably expressing human SDC4. Beads were washed, treated, and centrifuged as in A. Blots were detected with anti-SDC2 mAb L-18 or anti-SDC4 mAb N-19, respectively. A shows both 190- and 80-kD bands. B and C and all subsequent figures show the 80-kD band, which is more predominant in HEK293T transfectants.
Figure 3.
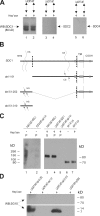
Lacritin's C terminus binds SDC1. (A) Schematic diagram of lacritin with dotted lines indicating N- and C-terminal truncations. All lacritin truncations were expressed as intein fusion proteins for affinity precipitation. The asterisk indicates mitogenic domain (Wang et al., 2006), and boxes represent PSIPRED-predicted α helices. (B) Lacritin-, C-5–, C-10–, C-15–, C-25–, and C-59–intein beads were incubated with lysates from HEK293T cells stably expressing human SDC1. Beads were washed and treated with heparitinase I/chondroitinase ABC. The digests were centrifuged, and pellets (P) and supernatants (S) blotted with mAb B-B4 for SDC1 core protein, all as in Fig. 2. (C) Incubation of lacritin-, N-15– and N-24–intein beads with the same human SDC1 lysates was followed with identical washing, heparitinase I/chondroitinase ABC digestion, centrifugation, and B-B4 mAb blotting. Lys, lysate.
Figure 4.
Lacritin-SDC1 binding is inhibited by soluble hS1ED, lacritin, and N-24, but not by C-25, C-59, HS, CS, SDC2, or SDC4. (A, top) Lacritin-intein beads were incubated with human SDC1 lysates from stably expressing HEK293T cells in the presence of increasing amounts of soluble HS (70–700 μg), HS (700 μg) plus CS (700 μg), or lacritin (14–700 μg) or no inhibitor (−). The quantity of soluble inhibitor was calibrated relative to the ∼7–8 μg of human SDC1 elutable from lacritin-intein beads with 1 M NaCl. After incubation, beads were washed extensively and treated with heparitinase I/chondroitinase ABC. The digests were centrifuged, and pellets were blotted with mAb B-B4 for SDC1 core protein, as in Fig. 2. (bottom) Lacritin-intein beads were incubated with human SDC1 lysates in the presence of soluble N-24, C-25, C-59 (14 μg of each), increasing amounts of bacterially expressed human SDC1 ectodomain (hS1ED; 35–700 μg), or with HEK293T cell–expressed native SDC2 or -4 (70 μg of each). Beads were washed and treated as above. (B) Quantification of inhibition binding. Error bars indicate SEM.
Figure 5.
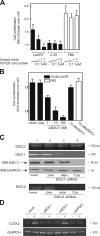
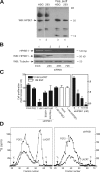
SDC1 is required for lacritin-dependent mitogenesis and COX2 expression. (A) Proliferation assay in which HSG cells were grown for 24 h in serum-free media containing 10 nM lacritin, 10 nM C-25 lacritin, or FBS in the absence or presence of increasing amounts of soluble hS1ED. (B) Identically performed proliferation assay in which HSG cells were treated with 10 nM lacritin or FBS 48 h after being mock transfected or transfected with 10 nM of Ambion's negative control siRNA #1 (neg), 1–100 nM SDC1 siRNA, or 10 nM SDC2 siRNA. Error bars indicate SEM. (C, top) RT-PCR and Western blotting of mock versus 10 nM SDC1 siRNA–treated cells. RT-PCR is for SDC1 and -2 mRNAs. Blotting is with mAb B-B4 for SDC1 core protein or with anti-GAPDH. (bottom) RT-PCR for SDC2 mRNA in mock-transfected cells or cells transfected with 10 nM SCD2 siRNA. (D) RT-PCR of COX2 expression by HSG cells without (−) or with (+) 10 nM lacritin stimulation. 48 h earlier, the cells were mock transfected or transfected with 10 nM SDC1, 10 nM SDC2, or 1 nM heparanase-1 (HPSE-1) siRNAs. At bottom is GAPDH expression.
Figure 6.
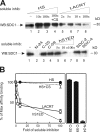
Lacritin and FGF2 bind different forms of cell surface SDC1. (A) Sequential affinity precipitation assays. Lanes 1–3 show lysate from human SDC1 stably expressing HEK293T cells sequentially incubated with three rounds of fresh FGF2-GST beads. Half of the final depleted lysate was then incubated with lacritin-intein beads (lane 4), and the other half was methanol precipitated (lane 9). Similarly, in lanes 5–7, a different aliquot of lysate from the same cells was sequentially incubated with three rounds of fresh lacritin-intein beads. Half of the final depleted lysate was then incubated with FGF2-GST beads (lane 8), and the other half was methanol precipitated (lane 10). Beads were washed and treated with heparitinase I/ chondroitinase ABC. The digests were centrifuged, and pellets (P) and supernatants (S) were blotted with mAb B-B4 for SDC1 core protein. Shown are digest supernatants (lanes 1–3 and 8) and pellets (lanes 4–7) as per heparitinase release of FGF2-bound or resistance of lacritin-bound SDC1, respectively. (B) HEK293T cells stably expressing human SDC1 were either lysed as usual or first briefly trypsinized (<5 min; 0.05%) and then treated with serum to inactivate trypsin, washed, and lysed. Both lysates were incubated with lacritin-intein beads. Beads were washed and treated with heparitinase I/chondroitinase ABC. The digests were centrifuged, and pellets (P) and supernatants (S) were blotted with mAb B-B4 for SDC1 core protein. (C) Lysates from HEK293T cells stably expressing human SDC1 were incubated with lacritin-intein beads. Beads were washed and either left untreated (lane 1) or treated with heparitinase I/chondroitinase ABC (lanes 2 and 3). The treated sample was centrifuged. Pellet (lane 2), supernatant (lane 3), untreated precipitate (lane 1), starting lysate (lane 4), and lacritin-intein solubilized from fresh lacritin-intein beads were blotted with mAb 3G10 for desaturated uronates in SDC1.
Figure 7.
Bacterial heparitinase digestion exposes FGF2-bindable SDC1 to lacritin binding via a domain in SDC1's N-terminal 50 amino acids. (A) Human SDC1 (lanes 1 and 2), SDC2 (lanes 3 and 4), and SDC4 (lanes 5 and 6) from stably expressing HEK293T cells were individually purified on FGF2-GST, eluted (0.5 and 1 M NaCl), treated with heparitinase I/chondroitinase ABC for 2 h, and incubated with lacritin-intein beads. Blotting is with mAb B-B4 for SDC1, polyclonal antibody L-18 for SDC2, or polyclonal N-19 for SDC4—all core protein specific. (B) Schematic diagram of human SDC1. The dotted line indicates truncation sites in the ectodomain forming the deletion constructs del 1–51, 51–252, and 51–310. Boxes represent PSIPRED-predicted α helices. Wavy lines represent HS and CS. TM, transmembrane domain. (C) Comparative incubation of FGF2-GST and lacritin-intein beads with human SDC1 or human SDC1 del 1–51 lysates from stably expressing HEK293T cells. After incubation, beads were washed extensively and either treated with heparitinase I/chondroitinase ABC (+) or left untreated (−). Beads were centrifuged, and pellets (P) and supernatants (S) were blotted with mAb B-B4 for SDC1 core protein. Lysate from HEK293T cells stably expressing SDC1 del 1–51 is blotted in lanes 6 and 7. (D) Comparative incubation of lacritin-intein beads with human SDC1 del 51–252, 1–51, or 51–310 lysates from stably or transiently expressing HEK293T cells. pcDNA is lysate from cells transfected with vector only. After incubation, beads were washed extensively and treated with heparitinase I/chondroitinase ABC. Beads were centrifuged, and pellets were blotted with mAb 3G10 for desaturated uronates in SDC1.
Figure 8.
Heparanase is expressed by HEK293T and HSG cells and is required for lacritin-dependent mitogenesis. (A) Lysates of HSG cells (lane 1) and HEK293T cells stably expressing human SDC1 (lanes 2) versus 2 M NaCl eluant of each after incubation with HiTrap heparin affinity columns (lanes 3 and 4, respectively). Blotting is with polyclonal anti–human heparanase-1 (HPSE1) antibody. (B) Lysates from HSG cells that had been mock transfected or transfected with 1 nM heparanase-1 siRNA. Blotting is with polyclonal anti–human HPSE1 or anti-tubulin antibodies. (C) Proliferation assay in which HSG cells were treated with 10 nM lacritin or 1 nM EGF 48 h after being mock transfected or transfected with 10 nM of Ambion's negative control siRNA #1 (neg), 1–100 nM HPSE1 siRNA, or 1 nM HPSE2 siRNA. Some HPSE1 siRNA cells were lacritin treated for 24 h in the presence of 1 μg of heparanase-enriched eluant (A) from HEK293T cells stably expressing SDC1 (1 nM + HPSE) or 0.0001 U of bacterial heparitinase. Error bars indicate SEM. (D) Sepharose CL-6B gel filtration chromatography of HS from lacritin and FGF2 affinity enriched SDC1 isolated from normal or HPSE1-depleted HSG cells. Lysates from cells labeled with 50 μCi/ml Na2 35SO4 in DME for 48 h were affinity precipitated with FGF2-GST or lacritin-intein. Equal microgram amounts of SDC1 bound to beads were digested with chondroitin ABC lyase to remove CS, eluted with 2 M NaCl, and subjected to NaBH4 eliminative cleavage. Released HS was neutralized by drop-wise addition of 1 M HCl and subjected to Sepharose CL-6B gel filtration chromatography to compare the relative size of HS chains. V0, void volume (dextran blue); Vt, total volume (sodium dichromate).
Figure 9.
Proposed model of epithelial cell targeting by lacritin. Deglycanated core protein of SDC1 targets the epithelial selective prosecretory mitogen lacritin. (i) Binding requires prior partial or complete removal of HS chains by endogenous HPSE1. (ii) Binding is mutually specified by lacritin's C-terminal mitogenic domain and SDC1's N terminus.
Similar articles
- Targeting of heparanase-modified syndecan-1 by prosecretory mitogen lacritin requires conserved core GAGAL plus heparan and chondroitin sulfate as a novel hybrid binding site that enhances selectivity.
Zhang Y, Wang N, Raab RW, McKown RL, Irwin JA, Kwon I, van Kuppevelt TH, Laurie GW. Zhang Y, et al. J Biol Chem. 2013 Apr 26;288(17):12090-101. doi: 10.1074/jbc.M112.422717. Epub 2013 Mar 15. J Biol Chem. 2013. PMID: 23504321 Free PMC article. - Loss of syndecan-1 and increased expression of heparanase in invasive esophageal carcinomas.
Mikami S, Ohashi K, Usui Y, Nemoto T, Katsube K, Yanagishita M, Nakajima M, Nakamura K, Koike M. Mikami S, et al. Jpn J Cancer Res. 2001 Oct;92(10):1062-73. doi: 10.1111/j.1349-7006.2001.tb01061.x. Jpn J Cancer Res. 2001. PMID: 11676857 Free PMC article. - Syndecan, a developmentally regulated cell surface proteoglycan that binds extracellular matrix and growth factors.
Bernfield M, Sanderson RD. Bernfield M, et al. Philos Trans R Soc Lond B Biol Sci. 1990 Mar 12;327(1239):171-86. doi: 10.1098/rstb.1990.0052. Philos Trans R Soc Lond B Biol Sci. 1990. PMID: 1969657 Review. - [Syndecans in cell adhesion and differentiation].
Brzóska E, Grabowska I. Brzóska E, et al. Postepy Biochem. 2005;51(1):52-9. Postepy Biochem. 2005. PMID: 16209342 Review. Polish.
Cited by
- Biosynthesized Multivalent Lacritin Peptides Stimulate Exosome Production in Human Corneal Epithelium.
Lee C, Edman MC, Laurie GW, Hamm-Alvarez SF, MacKay JA. Lee C, et al. Int J Mol Sci. 2020 Aug 26;21(17):6157. doi: 10.3390/ijms21176157. Int J Mol Sci. 2020. PMID: 32859014 Free PMC article. - Heparanase: busy at the cell surface.
Fux L, Ilan N, Sanderson RD, Vlodavsky I. Fux L, et al. Trends Biochem Sci. 2009 Oct;34(10):511-9. doi: 10.1016/j.tibs.2009.06.005. Epub 2009 Sep 3. Trends Biochem Sci. 2009. PMID: 19733083 Free PMC article. Review. - Heparanase stimulation of protease expression implicates it as a master regulator of the aggressive tumor phenotype in myeloma.
Purushothaman A, Chen L, Yang Y, Sanderson RD. Purushothaman A, et al. J Biol Chem. 2008 Nov 21;283(47):32628-36. doi: 10.1074/jbc.M806266200. Epub 2008 Sep 23. J Biol Chem. 2008. PMID: 18812315 Free PMC article. - Desmocollin-1 is associated with pro-metastatic phenotype of luminal A breast cancer cells and is modulated by parthenolide.
Lapcik P, Sulc P, Janacova L, Jilkova K, Potesil D, Bouchalova P, Müller P, Bouchal P. Lapcik P, et al. Cell Mol Biol Lett. 2023 Aug 24;28(1):68. doi: 10.1186/s11658-023-00481-6. Cell Mol Biol Lett. 2023. PMID: 37620794 Free PMC article. - Lacritin rescues stressed epithelia via rapid forkhead box O3 (FOXO3)-associated autophagy that restores metabolism.
Wang N, Zimmerman K, Raab RW, McKown RL, Hutnik CM, Talla V, Tyler MF 4th, Lee JK, Laurie GW. Wang N, et al. J Biol Chem. 2013 Jun 21;288(25):18146-61. doi: 10.1074/jbc.M112.436584. Epub 2013 May 2. J Biol Chem. 2013. PMID: 23640897 Free PMC article.
References
- Alexander, C.M., F. Reichsman, M.T. Hinkes, J. Lincecum, K.A. Becker, S. Cumberledge, and M. Bernfield. 2000. Syndecan-1 is required for Wnt-1-induced mammary tumorigenesis in mice. Nat. Genet. 25:329–332. - PubMed
- Barbosa, I., C. Morin, S. Garcia, A. Duchesnay, M. Oudghir, G. Jenniskens, H.Q. Miao, S. Guimond, G. Carpentier, J. Cebrian, et al. 2005. A synthetic glycosaminoglycan species during myogenesis. J. Cell Sci. 118:253–264. - PubMed
- Barden, J.A., R.M. Cuthbertson, W. Jia-Zhen, J.M. Moseley, and B.E. Kemp. 1997. Solution structure of parathyroid hormone related protein (residues 1-34) containing an Ala substituted for an Ile in position 15 (PTHrP[Ala15]-(1-34)). J. Biol. Chem. 272:29572–29578. - PubMed
- Beauvais, D.M., and A.C. Rapraeger. 2003. Syndecan-1-mediated cell spreading requires signaling by αvβ3 integrins in human breast carcinoma cells. Exp. Cell Res. 286:219–232. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous