Listeria monocytogenes flagella are used for motility, not as adhesins, to increase host cell invasion - PubMed (original) (raw)
Listeria monocytogenes flagella are used for motility, not as adhesins, to increase host cell invasion
Heather S O'Neil et al. Infect Immun. 2006 Dec.
Abstract
Flagellar structures contribute to the virulence of multiple gastrointestinal pathogens either as the effectors of motility, as adhesins, or as a secretion apparatus for virulence factors. Listeria monocytogenes is a food-borne, gram-positive pathogen that uses flagella to increase the efficiency of epithelial cell invasion (A. Bigot, H. Pagniez, E. Botton, C. Frehel, I. Dubail, C. Jacquet, A. Charbit, and C. Raynaud, Infect. Immun. 73:5530-5539, 2005; L. Dons, E. Eriksson, Y. Jin, M. E. Rottenberg, K. Kristensson, C. N. Larsen, J. Bresciani, and J. E. Olsen, Infect. Immun. 72:3237-3244, 2004). In this study, we aimed to elucidate the mechanism by which flagella contribute to L. monocytogenes invasion. To examine the role of flagella as adhesins, invasion and adhesion assays were performed with flagellated motile and nonmotile bacteria and nonflagellated bacteria. We observed that flagellated but nonmotile bacteria do not adhere to or invade human epithelial cells more efficiently than nonflagellated bacteria. These results indicated that flagella do not function as adhesins to enhance the adhesion of L. monocytogenes to targeted host cells. Instead, it appears that motility is important for tissue culture invasion. Furthermore, we tested whether motility contributes to early colonization of the gastrointestinal tract using a competitive index assay in which mice were infected orally with motile and nonmotile bacteria in a 1:1 ratio. Differential bacterial counts demonstrated that motile bacteria outcompete nonmotile bacteria in the colonization of the intestines at early time points postinfection. This difference is also reflected in invasion of the liver 12 h later, suggesting that flagellum-mediated motility enhances L. monocytogenes infectivity soon after bacterial ingestion in vivo.
Figures
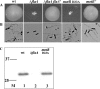
FIG. 1.
Characteristics of the wild-type and motility mutant strains. (A) Detection of motility. Strains of L. monocytogenes were stabbed into soft LB agar and incubated at room temperature for 36 h. A large area of bacterial growth is indicative of bacterial motility. (B) Detection of bacterium-associated flagella. Bacteria grown in BHI at 30°C with shaking were stained with crystal violet to visualize flagella by bright-field microscopy. (C) Immunodetection of flagellin from L. monocytogenes. Bacterial surface-extracted proteins were resolved by SDS-polyacrylamide gel electrophoresis, and flagellin was detected by Western immunoblotting. Lane M, prestained markers with molecular mass indicated in kDa on the left. wt, wild type.
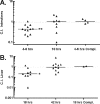
FIG. 2.
Motile bacteria outcompete nonmotile bacteria in initial colonization of the murine intestines and liver. Mice were infected orally with a 1:1 ratio of 10403S erm+ and Δ_flaA_, 10403S and Δ_flaA erm_+, or 10403S erm+ and Δ_flaA flaA_+ L. monocytogenes strains as described in detail in Materials and Methods. CIs were determined for colonization of the intestines (A) and liver (B) at indicated time points postinfection. Each data point represents the CI of one mouse. Data points were collected from a minimum of two independent experiments, except for the complemented (Compl.) Δ_flaA_ mutant strain, for which a single experiment was performed. A CI value of less than 1 indicates that the mutant strain was outcompeted by the wild-type strain. No differences were observed when mice were infected with 10403S erm+ and Δ_flaA_ versus 10403S and Δ_flaA erm_+ strains, indicating that the erythromycin resistance gene did not influence CI results. Horizontal bars indicate the median. Statistical differences from 1.0 were determined by Student's t test. *, P < 0.05; **, P ≤ 0.005.
Similar articles
- A Listeria adhesion protein-deficient Listeria monocytogenes strain shows reduced adhesion primarily to intestinal cell lines.
Jaradat ZW, Wampler JW, Bhunia AW. Jaradat ZW, et al. Med Microbiol Immunol. 2003 May;192(2):85-91. doi: 10.1007/s00430-002-0150-1. Epub 2002 Oct 19. Med Microbiol Immunol. 2003. PMID: 12736821 - The impact of growth history and flagellation on the adhesion of various Listeria monocytogenes strains to polystyrene.
Tresse O, Lebret V, Garmyn D, Dussurget O. Tresse O, et al. Can J Microbiol. 2009 Feb;55(2):189-96. doi: 10.1139/w08-114. Can J Microbiol. 2009. PMID: 19295651 - The role of L. monocytogenes serotype 4b gtcA in gastrointestinal listeriosis in A/J mice.
Faith N, Kathariou S, Cheng Y, Promadej N, Neudeck BL, Zhang Q, Luchansky J, Czuprynski C. Faith N, et al. Foodborne Pathog Dis. 2009 Jan-Feb;6(1):39-48. doi: 10.1089/fpd.2008.0154. Foodborne Pathog Dis. 2009. PMID: 18991548 - Listeria monocytogenes: a multifaceted model.
Hamon M, Bierne H, Cossart P. Hamon M, et al. Nat Rev Microbiol. 2006 Jun;4(6):423-34. doi: 10.1038/nrmicro1413. Nat Rev Microbiol. 2006. PMID: 16710323 Review. - The molecular mechanisms of actin-based intracellular motility by Listeria monocytogenes.
Chakraborty T. Chakraborty T. Microbiologia. 1996 Jun;12(2):237-44. Microbiologia. 1996. PMID: 8767707 Review.
Cited by
- Exploring the diversity of Listeria monocytogenes biofilm architecture by high-throughput confocal laser scanning microscopy and the predominance of the honeycomb-like morphotype.
Guilbaud M, Piveteau P, Desvaux M, Brisse S, Briandet R. Guilbaud M, et al. Appl Environ Microbiol. 2015 Mar;81(5):1813-9. doi: 10.1128/AEM.03173-14. Epub 2014 Dec 29. Appl Environ Microbiol. 2015. PMID: 25548046 Free PMC article. - RNA- and protein-mediated control of Listeria monocytogenes virulence gene expression.
Lebreton A, Cossart P. Lebreton A, et al. RNA Biol. 2017 May 4;14(5):460-470. doi: 10.1080/15476286.2016.1189069. Epub 2016 May 24. RNA Biol. 2017. PMID: 27217337 Free PMC article. Review. - Listeria monocytogenes Colonizes Pseudomonas fluorescens Biofilms and Induces Matrix Over-Production.
Puga CH, Dahdouh E, SanJose C, Orgaz B. Puga CH, et al. Front Microbiol. 2018 Jul 31;9:1706. doi: 10.3389/fmicb.2018.01706. eCollection 2018. Front Microbiol. 2018. PMID: 30108564 Free PMC article. - Flagellar motility is critical for Listeria monocytogenes biofilm formation.
Lemon KP, Higgins DE, Kolter R. Lemon KP, et al. J Bacteriol. 2007 Jun;189(12):4418-24. doi: 10.1128/JB.01967-06. Epub 2007 Apr 6. J Bacteriol. 2007. PMID: 17416647 Free PMC article. - Transcriptional and post-transcriptional regulation of the GmaR antirepressor governs temperature-dependent control of flagellar motility in Listeria monocytogenes.
Kamp HD, Higgins DE. Kamp HD, et al. Mol Microbiol. 2009 Oct;74(2):421-35. doi: 10.1111/j.1365-2958.2009.06874.x. Epub 2009 Oct 1. Mol Microbiol. 2009. PMID: 19796338 Free PMC article.
References
- Bishop, D. K., and D. J. Hinrichs. 1987. Adoptive transfer of immunity to Listeria monocytogenes. The influence of in vitro stimulation on lymphocyte subset requirements. J. Immunol. 139:2005-2009. - PubMed
- Dalton, C. B., C. C. Austin, J. Sobel, P. S. Hayes, W. F. Bibb, L. M. Graves, B. Swaminathan, M. E. Proctor, and P. M. Griffin. 1997. An outbreak of gastroenteritis and fever due to Listeria monocytogenes in milk. N. Engl. J. Med. 336:100-105. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases