An unconventional antigen translated by a novel internal ribosome entry site elicits antitumor humoral immune reactions - PubMed (original) (raw)
An unconventional antigen translated by a novel internal ribosome entry site elicits antitumor humoral immune reactions
Zeyu Xiong et al. J Immunol. 2006.
Abstract
Self-tumor Ags that elicit antitumor immune responses in responses to IFN-alpha stimulation remain poorly defined. We screened a human testis cDNA library with sera from three polycythemia vera patients who responded to IFN-alpha and identified a novel Ag, MPD6. MPD6 belongs to the group of cryptic Ags without conventional genomic structure and is encoded by a cryptic open reading frame located in the 3'-untranslated region of myotrophin mRNA. MPD6 elicits IgG Ab responses in a subset of polycythemia vera patients, as well as patients with chronic myelogenous leukemia and prostate cancer, suggesting that it is broadly immunogenic. The expression of myotrophin-MPD6 transcripts was up-regulated in some tumor cells, but only slightly increased in K562 cells in response to IFN-alpha treatment. By using bicistronic reporter constructs, we showed that the translation of MPD6 was mediated by a novel internal ribosome entry site (IRES) upstream of the MPD6 reading frame. Furthermore, the MPD6-IRES-mediated translation, but not myotrophin-MPD6 transcription, was significantly up-regulated in response to IFN-alpha stimulation. These findings demonstrate that a novel IRES-mediated mechanism may be responsible for the translation of unconventional self-Ag MPD6 in responsive to IFN-alpha stimulation. The eliciting antitumor immune response against unconventional Ag MPD6 in patients with myeloproliferative diseases suggests MPD6 as a potential target of novel immunotherapy.
Conflict of interest statement
Disclosures
The authors have no financial conflict of interest.
Figures
FIGURE 1
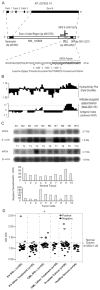
Molecular features of MPD6. A, Schematic representation of the location of unconventional Ag MPD6 gene in the 3′-UTR of myotrophin mRNA (GenBank accession no. NM_145808) as well as the genomic structure of the myotrophin-MPD6 gene locus (GenBank accession no. NT_007933.14). The MPD6 ORF (GenBank accession no. AY611627) is located in the region of bp 3061–3237 in the 3′-UTR of myotrophin, whereas the primary ORF myotrophin is located in the region of bp 229–585. An IRES is also located in the region of the 3′-UTR of myotrophin, bp 2855–2952 upstream of MPD6 ORF. B, Feature of MPD6 protein sequence. The MPD6 ORF encodes a 58-aa protein. The start codon is an unconventional start codon AUU (isoleucine), rather than the conventional start codon AUG (methionine). Hydrophilicity plot analysis using by Kyte-Dolittle method indicated that MPD6 has a C-terminal hydrophilic region, which corresponds to the region achieving the higher Jameson-Wolf antigenic index. The Jameson-Wolf antigenic index in the MPD6 C-terminal region is higher than that of previously characterized Abs recognized epitope threshold (mean − 2SD, 1.56). MPD6 peptide used in the ELISA was synthesized according to the MPD6 sequence from aa 39 to 58. C, Higher expression of myotrophin-MPD transcripts in some tumor cells detected by Northern blots. In the upper panel, the lanes N1 to N10 indicate various normal tissues in the order of brain (N1), liver (N2), placenta (N3), small intestine (N4), colon (N5), thymus (N6), spleen (N7), prostate (N8), testis (N9), and ovary (N10), respectively. In the middle panel, the lanes T1 to T10 indicate various tumor cells in the order of acute T cell leukemia (Jurkat cells) (T1), Burkitt’s lymphoma (CA46) (T2), breast cancer (MDA-MD-453) (T3), Burkitt’s lymphoma (Namalwa) (T4), epidermal carcinoma (A-431) (T5), uterine carcinoma (MES-SA) (T6), Burkitt’s lymphoma (Raji) (T7), osterosarcoma (MG-63) (T8), histiocytic lymphoma (U-937) (T9), and cervical adenocarcinoma (Hela S3) (T10), respectively. The hybridization analyses of the normal tissue and tumor cell expression (BD Clontech) with 32P-labeled specific probes, as indicated, were performed, respectively. The transcript sizes are indicated in kilobases. The ratio of the hybridization signal density of MPD6 transcript over the hybridization signal density of _β_-actin in the same sample was calculated as relative densitometric unit as presented in the lower panels. D, The IgG Ab responses to the C-terminal antigenic epitope (from the aa 39 to 58) of MPD6 detected by peptide ELISA. The experiments were repeated three times, and the representative results were shown. The mean + 2 SD of the OD405 ratios of the peptide over the coating control from 24 healthy donors were calculated as the upper limit of the normal range of Ab responses to MPD6 peptide (mean + 2 SD, 1.26). The detection rates of the IgG Ab responses to MPD6 peptide in the group of CML patients treating with IFN-α are statistically higher than that of CML patients treating with other therapies (_χ_2 goodness-of-fit test; *, p < 0.05).
FIGURE 2
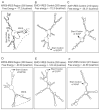
The predicted stem-loop structure in MPD6-IRES. The _cis_-acting regulatory elements in 3′-UTR were analyzed using the IRES website (〈
http://ifr31w3.toulouse.inserm.fr/IRESdatabase/
〉) and the UTR website (〈www2.ba.itb.cnr.it/UTRSite/〉) with generous support by Dr. S. Liuni at the Bioinformatics and Genomic Group in Italy. The secondary structures of MPD6-IRES (A and D), EMCV-IRES (B and E), and XIAP-IRES (C and F) were predicted by using two web-based algorithms, MFOLD-Zuker (〈
www.bioinfo.rpi.edu/applications/mfold/old/rna/
〉) (A–C) and RNAfold (〈
〉) (D–F). The free energy of the secondary structure of these IRES regions was also calculated with both algorithms. The start codon and IRES region of MPD6 are indicated.
FIGURE 3
Upregulated translation of EGFP directed by MPD6-IRES in response to IFN-α stimulation. A, Following bicistrionic vectors with DsRed as the upstream primary ORF and with EGFP as the downstream secondary ORF were constructed (upper left panel), including a complete sequence (myotrophin 3′-UTR plus MPD6-IRES) between myotrophin ORF and MPD6 ORF placed between DsRed ORF and EGFP ORF (A1); a deletion mutant with the 5′ portion of the myotrophin 3′-UTR but having no MPD6-IRES sequence in between DsRed ORF and EGFP ORF (A2); and a bicistronic vector control with EMCV-IRES as an documented IRES positive control (A3). The nontransfected cells were used as a negative control for DsRed and EGFP expression (data not shown). In the upper right panels, the bivariate plots show the DsRed fluorescence-positive cells (ordinate) and the EGFP fluorescence-positive cells (abscissa), and percentages of cells in each quadrant in the K562 cells stably transfected with each bicistronic reporter construct, and stimulated with IFN-α for 0, 10, and 24 h. The experiments were repeated three times, and representative results are shown. In the lower right panel, the confidential intervals (mean ± 2SD, 0.81–1.36; shown in the dashed lines) of the ratio of the percentage of DsRed-positive cells after IFN-α stimulation over that before IFN-α stimulation were generated. Similarly, in the lower left panel, the ratio of the percentage of EGFP-positive cells after IFN-α stimulation over that before IFN-α stimulation were calculated. The ratio of the percentage of EGFP-positive cells in MPD6-IRES transfected group 24 h after IFN-α stimulation over that before IFN-α stimulation was 2.3, which was significantly higher than the upper limit (1.36) of the DsRed confidential intervals (p < 0.05). B, The expression of eIF-2_α_ and phosphorylated eIF-2_α_ in K562 myeloid leukemia cells stimulated with IFN-α detected by specific Abs. The upregulation of the expression of eIF-2_α_ and phosphorylated eIF-2_α_ in responses to IFN-α stimulation in myeloid leukemia K562 cells was measured by Western blots with specific Abs to eIF-2_α_ and phosphorylated eIF-2_α_, respectively, at the time points as indicated. The Western blot with Ab to β_-actin was performed as a housekeeping protein control and a no-response control for IFN-α stimulation. In the right panel, the relative densitometric units were calculated by normalizing the densities of the eIF-2_α and phosphorylated eIF-2_α_ with that of β_-actin in the same sample. The relative densitometric units for the expression of eIF-2_α and phosphorylated eIF-2_α_ after IFN-α stimulation over that before IFN-α stimulation were calculated and shown as the percentages. C, The MPD6 expression in K562 myeloid leukemia cells stimulated with IFN-α detected by semiquantitative RT-PCR. The upregulation of MPD6 transcripts in responses to IFN-α stimulation in myeloid leukemia K562 cells was measured by semiquantitative RT-PCR at the time points as indicated (left panel). The RT-PCR amplification of _β_-actin transcripts was performed as a housekeeping gene control for RT-PCR and a no-response control for IFN-α stimulation. The RT-PCR amplification of ISG15 was used as a positive control for IFN-α stimulation. In the right panel, the relative densitometric units were calculated by normalizing the densities of the PCR products of MPD6 and ISG15 with that of _β_-actin in the same sample. The relative densitometric units for the expression of myotrophin/MPD6 and ISG15 transcripts after IFN-α stimulation over that before IFN-α stimulation were calculated and shown as the percentages. D, The proposed working model of MPD6 translation mediated by MPD6-IRES. MPD6-IRES is found to be capable in mediation of upregulated translation of MPD6 in response to IFN-α stimulation, which may result from IFN-α_-mediated phosphorylation of eIF-2_α.
Similar articles
- Novel tumor antigens elicit anti-tumor humoral immune reactions in a subset of patients with polycythemia vera.
Xiong Z, Yan Y, Liu E, Silver RT, Verstovsek S, Yang F, Wang H, Prchal J, Yang XF. Xiong Z, et al. Clin Immunol. 2007 Mar;122(3):279-87. doi: 10.1016/j.clim.2006.10.006. Epub 2006 Nov 17. Clin Immunol. 2007. PMID: 17113348 Free PMC article. - A novel unconventional antigen MPD5 elicits anti-tumor humoral immune responses in a subset of patients with polycythemia vera.
Xiong Z, Liu E, Yan Y, Silver RT, Yang F, Chen IH, Hodge I, Verstovsek S, Segura FJ, Wang H, Prchal J, Yang XF. Xiong Z, et al. Int J Immunopathol Pharmacol. 2007 Apr-Jun;20(2):373-80. doi: 10.1177/039463200702000218. Int J Immunopathol Pharmacol. 2007. PMID: 17624250 Free PMC article. - Identification of a novel human cancer/testis antigen, KM-HN-1, recognized by cellular and humoral immune responses.
Monji M, Nakatsura T, Senju S, Yoshitake Y, Sawatsubashi M, Shinohara M, Kageshita T, Ono T, Inokuchi A, Nishimura Y. Monji M, et al. Clin Cancer Res. 2004 Sep 15;10(18 Pt 1):6047-57. doi: 10.1158/1078-0432.CCR-04-0475. Clin Cancer Res. 2004. PMID: 15447989 - [Use of interferon in the treatment of chronic myeloproliferative disorders].
Robak T. Robak T. Acta Haematol Pol. 1992;23(2 Suppl 1):30-7. Acta Haematol Pol. 1992. PMID: 1488870 Review. Polish. - Targeting internal ribosome entry site (IRES)-mediated translation to block hepatitis C and other RNA viruses.
Dasgupta A, Das S, Izumi R, Venkatesan A, Barat B. Dasgupta A, et al. FEMS Microbiol Lett. 2004 May 15;234(2):189-99. doi: 10.1016/j.femsle.2004.03.045. FEMS Microbiol Lett. 2004. PMID: 15135522 Review.
Cited by
- Assessment of ApoC1, LuzP6, C12orf75 and OCC-1 in cystic glioblastoma using MALDI-TOF mass spectrometry, immunohistochemistry and qRT-PCR.
Evangelou P, Groll M, Oppermann H, Gaunitz F, Eisenlöffel C, Müller W, Eschrich K, Schänzer A, Nestler U. Evangelou P, et al. Med Mol Morphol. 2019 Dec;52(4):217-225. doi: 10.1007/s00795-019-00223-8. Epub 2019 Apr 20. Med Mol Morphol. 2019. PMID: 31006040 Free PMC article. - Interferon-alpha targets JAK2V617F-positive hematopoietic progenitor cells and acts through the p38 MAPK pathway.
Lu M, Zhang W, Li Y, Berenzon D, Wang X, Wang J, Mascarenhas J, Xu M, Hoffman R. Lu M, et al. Exp Hematol. 2010 Jun;38(6):472-80. doi: 10.1016/j.exphem.2010.03.005. Epub 2010 Mar 18. Exp Hematol. 2010. PMID: 20303384 Free PMC article. - Novel tumor antigens elicit anti-tumor humoral immune reactions in a subset of patients with polycythemia vera.
Xiong Z, Yan Y, Liu E, Silver RT, Verstovsek S, Yang F, Wang H, Prchal J, Yang XF. Xiong Z, et al. Clin Immunol. 2007 Mar;122(3):279-87. doi: 10.1016/j.clim.2006.10.006. Epub 2006 Nov 17. Clin Immunol. 2007. PMID: 17113348 Free PMC article. - Duocarmycin SA Reduces Proliferation and Increases Apoptosis in Acute Myeloid Leukemia Cells In Vitro.
Chen WA, Williams TG, So L, Drew N, Fang J, Ochoa P, Nguyen N, Jawhar Y, Otiji J, Duerksen-Hughes PJ, Reeves ME, Casiano CA, Jin H, Dovat S, Yang J, Boyle KE, Francis-Boyle OL. Chen WA, et al. Int J Mol Sci. 2024 Apr 14;25(8):4342. doi: 10.3390/ijms25084342. Int J Mol Sci. 2024. PMID: 38673926 Free PMC article. - An additional ORF on meloe cDNA encodes a new melanoma antigen, MELOE-2, recognized by melanoma-specific T cells in the HLA-A2 context.
Godet Y, Moreau-Aubry A, Mompelat D, Vignard V, Khammari A, Dreno B, Lang F, Jotereau F, Labarriere N. Godet Y, et al. Cancer Immunol Immunother. 2010 Mar;59(3):431-9. doi: 10.1007/s00262-009-0762-z. Cancer Immunol Immunother. 2010. PMID: 19730858 Free PMC article.
References
- Belardelli F, Ferrantini M, Proietti E, Kirkwood JM. Interferon-α in tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002;13:119–134. - PubMed
- Liu E, Jelinek J, Pastore YD, Guan Y, Prchal JF, Prchal JT. Discrimination of polycythemias and thrombocytoses by novel, simple, accurate clonality assays and comparison with PRV-1 expression and BFU-E response to erythropoietin. Blood. 2003;101:3294–3301. - PubMed
- Lengfelder E, Berger U, Hehlmann R. Interferon-α in the treatment of polycythemia vera. Ann Hematol. 2000;79:103–109. - PubMed
- Fujii S. Role of interferon-α and clonally expanded T cells in the immunotherapy of chronic myelogenous leukemia. Leuk Lymphoma. 2000;38:21–38. - PubMed
- Sacchi S, Kantarjian H, O’Brien S, Cohen PR, Pierce S, Talpaz M. Immune-mediated and unusual complications during interferon-alfa therapy in chronic myelogenous leukemia. J Clin Oncol. 1995;13:2401–2407. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases