Protecting axonal degeneration by increasing nicotinamide adenine dinucleotide levels in experimental autoimmune encephalomyelitis models - PubMed (original) (raw)
Comparative Study
Protecting axonal degeneration by increasing nicotinamide adenine dinucleotide levels in experimental autoimmune encephalomyelitis models
Shinjiro Kaneko et al. J Neurosci. 2006.
Abstract
Axonal damage is a major morphological alteration in the CNS of patients with multiple sclerosis (MS) and its animal model, experimental autoimmune encephalomyelitis (EAE). However, the underlying mechanism for the axonal damage associated with MS/EAE and its contribution to the clinical symptoms remain unclear. The expression of a fusion protein, named "Wallerian degeneration slow" (Wld(S)), can protect axons from degeneration, likely through a beta-nicotinamide adenine dinucleotide (NAD)-dependent mechanism. In this study, we find that, when induced with EAE, Wld(S) mice showed a modest attenuation of behavioral deficits and axon loss, suggesting that EAE-associated axon damage may occur by a mechanism similar to Wallerian degeneration. Furthermore, nicotinamide (NAm), an NAD biosynthesis precursor, profoundly prevents the degeneration of demyelinated axons and improves the behavioral deficits in EAE models. Finally, we demonstrate that delayed NAm treatment is also beneficial to EAE models, pointing to the therapeutic potential of NAm as a protective agent for EAE and perhaps MS patients.
Figures
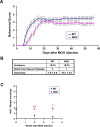
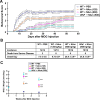
Figure 1.
Wlds modestly delayed the onset and attenuated the behavioral deficits of EAE. A, Behavioral scores (mean ± SEM) of EAE in C57BL/6 or Wld s mice. Differences between these groups were significant as determined by two-tailed Student's t test, p < 0.05 from 6 d p.i. B, Clinical features of EAE in C57BL/6 or Wld s mice. The onset was significantly delayed in Wld s mice when compared with the wild-type group (*p < 0.01; Student's t test). C, NAD levels (presented as mean ± SEM) of the cervical spinal cords of EAE animals as analyzed by HPLC. In C57BL/6 mice, NAD levels at 2 or 4 weeks p.i. were significantly decreased compared with uninduced controls (p = 0.0012, p = 0.0004 at 2 and 4 weeks p.i., respectively). However, the NAD levels in Wld s mice were preserved compared with those from wild-type EAE mice (**p < 0.001, *p < 0.01 at 2 and 4 weeks p.i., respectively; Student's t test).
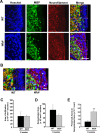
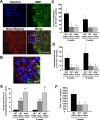
Figure 2.
Wlds expression reduces axon loss at 8 weeks p.i. A, Representative images of transverse sections from EAE-induced wild-type (top panels) and Wld s (bottom panels) mice at 8 weeks p.i. stained with Hoechst 33258 (left), anti-MBP (middle), or anti-NF (right). B, The demyelinated areas are enlarged and the arrowheads indicate preserved axons in the demyelinated area. Scale bars: A, 30 μm; B, 10 μm. C–E, Quantification of the average areas of infiltration (p = 0.99) (C) and demyelination (p = 0.39) (D) per section, which showed no significant difference, and the average numbers of NF+/MBP− fibers in demyelinated areas, which was significantly increased in Wld s mice when compared with wild type (**p < 0.001; Student's t test) (E). Areas of infiltration (×103 μm2/section) or demyelination (×103 μm2/section) were determined as the measured Hoescht+ or MBP− areas averaged from 10 transverse sections per animal for each group. The number of preserved axons in demyelinated areas (×10−3/μm2) was determined by counting the number of NF+ puncta in demyelinated MBP− regions, and then dividing by the area of demyelination. Error bars indicate SEM.
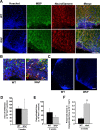
Figure 3.
Wlds expression reduces axon loss at 2 weeks p.i. A–C, Representative images of transverse sections from the lumbar spinal cords of EAE-induced wild-type or Wld s mice at 2 weeks p.i. Images of sections stained with Hoechst 33258/anti-MBP/NF (A, B) show a similar extent of infiltration, but significantly more NF+ fibers in the infiltrated and demyelinated areas from Wld s EAE mice compared with wild-type EAE mice. Images in B are enlarged from the indicated areas in A. The arrowheads indicate several preserved axons in the lesion areas. Low magnification images of Hoechst 33258 staining indicating similar extents of infiltration in both wild-type and Wld s mice induced with EAE (C). Scale bars: A, C, 100 μm; B, 10 μm. D–F, Quantification of the average areas of infiltration (p = 0.97) (D) and demyelination (p = 0.25) (E) per section, which showed no significant difference, and the average numbers of NF+/MBP− fibers in demyelinated areas, which was significantly increased in Wld s mice compared with wild type (**p < 0.001; Student's t test) (F). Error bars indicate SEM.
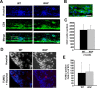
Figure 4.
A–C, Representative images of sagittal sections from the lumbar spinal cords from wild type (A, B) and Wld s (C) mice induced with EAE at 2 weeks p.i. The sections were stained with Hoechst 33258, anti-MBP, or -NF. Significantly more NF+ fibers were observed in the infiltrated and demyelinated areas from Wld s mice than from wild-type animals. Note that a residual demyelinated NF+ axonal ending is shown by the arrow in B. Scale bars, 10 μm. D, Electron micrographs of cervical spinal cord tissue near the lesion sites in the control group of EAE models at 2 weeks, or 8 weeks p.i. Note the large numbers of degenerating axons in both acute and chronic phases of EAE. The right panels show higher magnifications of degenerating axons in the left panel at each time point. Scale bars: left panels, 10 μm; enlarged right panels, 5 μm, at each time point.
Figure 5.
The effects of Wlds on infiltration of immune cells and cell death in the EAE model. Sagittal sections from 2 weeks p.i. EAE-induced wild-type or Wld s mice were stained with Hoechst 33258 and antibodies against CD4 (A). Representative images are shown in A. The panel shown in B is an enlargement of the selected area of merged image. Scale bars: A, 25 μm; enlarged panel, 10 μm. Quantification of the average number of infiltrated CD4+ (C) cells (numbers per section) from six sections per animals, eight animals per group, showing no significant differences in these groups (p = 0.97; Student's t test). D, Representative images of TUNEL (red in bottom panel) costained with Hoechst 33258 (white in top panel and blue in bottom panel) in the lesion areas of the cervical spinal cord transverse sections of wild-type (left panels) or Wld s (right panels) mice at 2 weeks p.i. Scale bars, 15 μm. E, Quantification of TUNEL-positive cells in EAE-induced wild-type and Wld s mice (10 sections per animal from 8 animals in each group) at 2 weeks p.i., showing no significant difference in these groups (p = 0.93; Student's t test). Error bars indicate SEM.
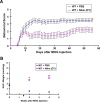
Figure 6.
Profound protective effects of NAm on the behavioral defects of the EAE model. A, Behavioral scores (mean ± SEM) of different groups of EAE mice. Differences between each treated group and untreated controls were significant as determined by two-tailed Student's t test: p < 0.05, from 7 d p.i. in the low-dose NAm-treated wild-type group (125 mg/kg); p < 0.001, from 6 d p.i. in the high-dose NAm-treated wild-type and Wld s groups (500 mg/kg). B, Clinical features of the EAE model in NAm-treated groups and untreated controls. Symptom onset was significantly delayed in each treated group when compared with untreated wild-type EAE mice (*p < 0.05; **p < 0.001; Student's t test). C, NAD levels (mean ± SEM) in cervical spinal cords from different groups of EAE mice as analyzed by HPLC. At both 2 and 4 weeks p.i., the NAD levels in each treated group were significantly higher than those from untreated controls (**p < 0.001; Student's t test).
Figure 7.
Profound protective effects of NAm on inflammation, demyelination and axonal loss in the EAE model. A, B, Representative images showing the effects of NAm treatment on infiltration, demyelination, and axonal loss. Transverse sections from 8 weeks p.i. EAE-induced Wld s mice treated with high-dose NAm were stained with Hoechst 33258 and antibodies against MBP and NF (A). Higher magnification of the merged image is shown in B. Scale bars: A, 50 μm; B, 10 μm. C–E, Quantification of average areas of infiltration (C) and demyelination (D), as well as average number of NF+/MBP− fibers in demyelinated areas (E). Both NAm-treated groups showed a significant reduction in infiltration at 2 weeks p.i. (*p < 0.05), and demyelination at both 2 and 8 weeks p.i. (*p < 0.05), but not infiltration at 8 weeks p.i. (p = 0.11, p = 0.10 in wild-type and Wld s mice, respectively) (C, D). Average numbers of preserved fibers in demyelinated areas were also significantly increased in both NAm-treated groups at both time points (**p < 0.001; Student's t test) (E). Quantification of the infiltrated CD4+ cells (F), showing a significant reduction of CD4+ cellular infiltration in both NAm-treated groups (*p < 0.05; Student's t test). Average immune cell numbers per section from six sections of each animal, eight animals per group, were quantified. Error bars indicate SEM.
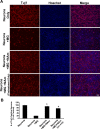
Figure 8.
NAm attenuates neurotoxicity induced by LPS-activated microglia. A, Representative images showing cocultures of E18 cortical neurons and LPS-activated microglia in different conditions stained with Tuj-1 antibody or Hoechst 33258. Images of cultured neurons without microglia (first row), with microglia (second row), with microglia plus 25 m
m
NAm (third row), and with microglia plus 25 m
m
NAm (pretreatment only) (fourth row). Scale bar, 100 μm. B, Quantification of Tuj-1-positively stained neurons under different conditions. NAm treatments (both continuous or pretreatment alone) significantly preserved neurons cocultured with LPS-activated microglia (*p < 0.001; Student's t test). Error bars indicate SEM.
Figure 9.
Protective effects of delayed NAm treatment on behavioral defects in the EAE model. A, Behavioral scores (mean ± SEM) of EAE in untreated (n = 14) or delayed treated (DT) (n = 12) groups. Differences between these groups were significant as determined by two-tailed Student's t test (p < 0.01 from 13 d p.i.). The differences seen before 10 d p.i. are attributable to the death of three mice in the untreated group when they showed clear behavioral deficits. B, NAD levels (mean ± SEM) of cervical spinal cords from untreated or delayed treated groups. At both 2 and 4 weeks p.i., the NAD levels in the treated groups were significantly higher than those in untreated animals (*p < 0.05; **p < 0.001; Student's t test). The arrows indicate the starting point of delayed treatment in both A and B.
Figure 10.
Protective effects of delayed NAm treatment on inflammation, demyelination, and axonal loss in the EAE model. A, B, Representative images showing the effects of delayed NAm treatment on infiltration, demyelination, and axonal loss. Transverse sections from wild-type EAE animals with delayed NAm treatment at 8 weeks p.i. were stained with Hoechst 33258 and antibodies against MBP and NF (A). Higher magnification of the merged image is shown in B. The arrowheads indicate several preserved axons in the demyelinated lesions. Scale bars: A, 50 μm; B, 10 μm. C–E, Quantification of the average areas of infiltration (p = 0.65) (C) or demyelination (p = 0.14) (D) showed no significant difference, but the average number of NF+/MBP− fibers in demyelinated areas was increased in animals with delayed treatment with NAm (*p < 0.05; Student's t test) (E). Error bars indicate SEM.
Similar articles
- Protective effects of progesterone administration on axonal pathology in mice with experimental autoimmune encephalomyelitis.
Garay L, Gonzalez Deniselle MC, Meyer M, Costa JJ, Lima A, Roig P, De nicola AF. Garay L, et al. Brain Res. 2009 Aug 4;1283:177-85. doi: 10.1016/j.brainres.2009.04.057. Epub 2009 Jun 2. Brain Res. 2009. PMID: 19497309 - Relationship of acute axonal damage, Wallerian degeneration, and clinical disability in multiple sclerosis.
Singh S, Dallenga T, Winkler A, Roemer S, Maruschak B, Siebert H, Brück W, Stadelmann C. Singh S, et al. J Neuroinflammation. 2017 Mar 17;14(1):57. doi: 10.1186/s12974-017-0831-8. J Neuroinflammation. 2017. PMID: 28302146 Free PMC article. - A local mechanism mediates NAD-dependent protection of axon degeneration.
Wang J, Zhai Q, Chen Y, Lin E, Gu W, McBurney MW, He Z. Wang J, et al. J Cell Biol. 2005 Aug 1;170(3):349-55. doi: 10.1083/jcb.200504028. Epub 2005 Jul 25. J Cell Biol. 2005. PMID: 16043516 Free PMC article. - Neuroprotective strategies in MS: lessons from C57BL/Wld(S) mice.
Coleman MP, Adalbert R, Beirowski B. Coleman MP, et al. J Neurol Sci. 2005 Jun 15;233(1-2):133-8. doi: 10.1016/j.jns.2005.03.028. J Neurol Sci. 2005. PMID: 15899498 Review. - Molecular mechanisms in the initiation phase of Wallerian degeneration.
Chang B, Quan Q, Lu S, Wang Y, Peng J. Chang B, et al. Eur J Neurosci. 2016 Aug;44(4):2040-8. doi: 10.1111/ejn.13250. Epub 2016 May 30. Eur J Neurosci. 2016. PMID: 27062141 Review.
Cited by
- Diapause formation and downregulation of insulin-like signaling via DAF-16/FOXO delays axonal degeneration and neuronal loss.
Calixto A, Jara JS, Court FA. Calixto A, et al. PLoS Genet. 2012;8(12):e1003141. doi: 10.1371/journal.pgen.1003141. Epub 2012 Dec 27. PLoS Genet. 2012. PMID: 23300463 Free PMC article. - Neuronal death induced by misfolded prion protein is due to NAD+ depletion and can be relieved in vitro and in vivo by NAD+ replenishment.
Zhou M, Ottenberg G, Sferrazza GF, Hubbs C, Fallahi M, Rumbaugh G, Brantley AF, Lasmézas CI. Zhou M, et al. Brain. 2015 Apr;138(Pt 4):992-1008. doi: 10.1093/brain/awv002. Epub 2015 Feb 11. Brain. 2015. PMID: 25678560 Free PMC article. - Sarm1 knockout protects against early but not late axonal degeneration in experimental allergic encephalomyelitis.
Viar K, Njoku D, Secor McVoy J, Oh U. Viar K, et al. PLoS One. 2020 Jun 25;15(6):e0235110. doi: 10.1371/journal.pone.0235110. eCollection 2020. PLoS One. 2020. PMID: 32584865 Free PMC article. - NAD+ and SIRT3 control microtubule dynamics and reduce susceptibility to antimicrotubule agents.
Harkcom WT, Ghosh AK, Sung MS, Matov A, Brown KD, Giannakakou P, Jaffrey SR. Harkcom WT, et al. Proc Natl Acad Sci U S A. 2014 Jun 17;111(24):E2443-52. doi: 10.1073/pnas.1404269111. Epub 2014 Jun 2. Proc Natl Acad Sci U S A. 2014. PMID: 24889606 Free PMC article. - Stem Cell Therapies for Progressive Multiple Sclerosis.
Smith JA, Nicaise AM, Ionescu RB, Hamel R, Peruzzotti-Jametti L, Pluchino S. Smith JA, et al. Front Cell Dev Biol. 2021 Jul 9;9:696434. doi: 10.3389/fcell.2021.696434. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34307372 Free PMC article. Review.
References
- Adelman B, Sandrock A, Panzara MA. Natalizumab and progressive multifocal leukoencephalopathy. N Engl J Med. 2005;353:432–433. - PubMed
- Aktas O, Smorodchenko A, Brocke S, Infante-Duarte C, Topphoff US, Vogt J, Prozorovski T, Meier S, Osmanova V, Pohl E, Bechmann I, Nitsch R, Zipp F. Neuronal damage in autoimmune neuroinflammation mediated by the death ligand TRAIL. Neuron. 2005;46:421–432. - PubMed
- Araki T, Sasaki Y, Milbrandt J. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science. 2004;305:1010–1013. - PubMed
- Berger F, Ramirez-Hernandez MH, Ziegler M. The new life of a centenarian: the new life of a centenarian: signalling functions of NAD(P) Trends Biochem Sci. 2004;29:111–118. - PubMed
- Bjartmar C, Wujek JR, Trapp BD. Axonal loss in the pathology of MS: consequences for understanding the progressive phase of the disease. J Neurol Sci. 2003;206:165–171. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases