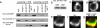Recruitment of dynein to the Jurkat immunological synapse - PubMed (original) (raw)
Recruitment of dynein to the Jurkat immunological synapse
Jeffrey Combs et al. Proc Natl Acad Sci U S A. 2006.
Abstract
Binding of T cells to antigen-presenting cells leads to the formation of the immunological synapse, translocation of the microtubule-organizing center (MTOC) to the synapse, and focused secretion of effector molecules. Here, we show that upon activation of Jurkat cells microtubules project from the MTOC to a ring of the scaffolding protein ADAP, localized at the synapse. Loss of ADAP, but not lymphocyte function-associated antigen 1, leads to a severe defect in MTOC polarization at the immunological synapse. The microtubule motor protein cytoplasmic dynein clusters into a ring at the synapse, colocalizing with the ADAP ring. ADAP coprecipitates with dynein from activated Jurkat cells, and loss of ADAP prevents MTOC translocation and the specific recruitment of dynein to the synapse. These results suggest a mechanism that links signaling through the T cell receptor to translocation of the MTOC, in which the minus end-directed motor cytoplasmic dynein, localized at the synapse through an interaction with ADAP, reels in the MTOC, allowing for directed secretion along the polarized microtubule cytoskeleton.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
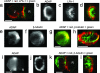
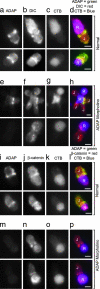
ADAP, LFA-1, and microtubules at the Jurkat immunological synapse. (a_–_d) SEE-coated Raji (R) cells conjugated to Jurkat (J) cells were fixed and immunostained for LFA-1 and ADAP. The _z_-axis stacks (256 images) were acquired for LFA-1, and ADAP fluorescence data were processed as described (3). A Jurkat–Raji pair immunostained for ADAP (red) and LFA-1 (green) is shown from a side view (a) and facing the synapse with separate panels for ADAP (b) and LFA-1 (c). A red–green overlay of the data from b and c is shown in d. Note that the ADAP and LFA-1 rings are separate and distinct. (e_–_g) Jurkat cells were immunostained for ADAP (e) and tubulin (f) with the red–green overlay shown in g (red, ADAP; green, tubulin). (h) SEE-coated Raji cells pretreated with colchicine to depolymerize microtubules were mixed with normal Jurkat cells and then immunostained for tubulin (green) and ADAP (red). Image stacks were acquired and processed as for a_–_d. A typical Jurkat–Raji pair shows microtubules projecting from the MTOC to the ADAP ring where the microtubules often closely follow its surface. A rotatable view is available as Movie 1. (i_–_j) Cells were prepared identically to the procedure in a_–_d except that Jurkat cells were pretreated with 10 μM colchicine to depolymerize the microtubules. The results show that the ADAP ring forms in the absence of an intact microtubule cytoskeleton. (k and l) Cells were prepared identically to the procedure in a_–_d except that JB2.7 (LFA-1-deficient) Jurkat cells were used in place of normal Jurkat cells. The results show that microtubules are associated with a typical ADAP ring in the absence of LFA-1. Data are representative of two to three independent experiments. (Scale bars: 5 μm.)
Fig. 2.
A dynein complex clusters at the immunological synapse and associates with ADAP. (a) To determine whether ADAP is bound to dynein, rabbit anti-DIC 1467 was used to immunoprecipitate (IP) dynein from homogenates of SEE-stimulated or unstimulated Jurkat–Raji mixtures. The immunoprecipitate was then probed on separate blots with mouse anti-ADAP mAb and mouse anti-DIC mAb 70.1. The results show an increase in both ADAP (Top) and dynein (Middle) in the activated compared with nonactivated Jurkat lysates. (Bottom) Levels of antibody in the IP are shown. No ADAP or dynein is detected when specific antibody is replaced with beads alone or beads bound to rabbit anti-mouse Ig. Similar amounts of total dynein and ADAP were detected in the whole-cell lysates (WCL). (b) The whole-cell lysates from a were also probed with anti-DIC 1467. The results show that 1467 recognizes similar levels of dynein in the activated and nonactivated Jurkat lysates. (c and d) Computerized 3D reconstructions of fluorescence data were prepared from Jurkat/SEE-coated Raji conjugates fixed and immunostained with anti-DIC mAb 70.1 (c) or rabbit anti-DIC 1467 (d). Both antibodies show a ring-like staining pattern at the Jurkat-Raji immunological synapse. (e and f) The same procedures as in c and d were then used to examine Jurkat–Raji pairs immunostained for ADAP (e) and dynein with mAb 70.1 (f). (g) The red-green-blue overlay shows DIC colocalizes with ADAP at the synapse. Data are representative of three independent experiments. (Scale bars: 5 μm.)
Fig. 3.
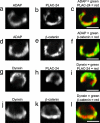
The spatial relationship between ADAP, dynein, PLAC-24, and β-catenin at the synapse. Jurkat–Raji cell pairs were fixed, immunostained, and subsequently processed by computerized 3D reconstruction as described. (a_–_c) A Jurkat–Raji pair is immunostained for ADAP (a) and PLAC-24 (b) with the red–green overlay shown in c. (d_–_f) A Jurkat–Raji pair is immunostained for ADAP (d) and β-catenin (e) with the red–green overlay shown in f. (g_–_i) A Jurkat–Raji pair is immunostained for dynein using mAb 70.1 (g) and PLAC-24 (h) with the red–green overlay shown in i. (j_–_l) A Jurkat–Raji pair is immunostained for dynein using mAb 70.1 (j) and β-catenin (k) with the red–green overlay shown in l. Data are representative of three independent experiments. (Scale bars: 5 μm.)
Fig. 4.
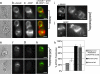
MO-mediated ADAP knockdown abolishes MTOC polarization in Jurkat cells. Jurkat cells were electroporated with either an antisense MO directed against ADAP (ADAP MO) or a standard control MO 24 h before experiment. Normal (untreated), ADAP MO, and control MO Jurkat cells were used in the preparation of Jurkat (J)–Raji (R) cell pairs as described. (a_–_d) Normal Jurkat–Raji pairs shown in bright field (a) were immunostained for α-tubulin (b) and ADAP (mouse anti-fyb, clone 5) (c) with the red–green overlay shown in d. The results show typical ADAP clustering and MTOC polarization. (e_–_h) ADAP MO-treated Jurkat–Raji pairs prepared as in a_–_d show a loss of ADAP from the synapse and failure of MTOC polarization. (i_–_j) Control MO prepared as in a_–_d show normal ADAP clustering and normal MTOC polarization. (k) A bar graph summarizing polarization counts demonstrates a dramatic reduction in MTOC polarization in the ADAP MO Jurkat cells compared with normal and control MO Jurkat cell preparations. Note that background polarization levels obtained by treating Jurkat cells with the Srk kinase inhibitor PP2 are 25%. Data are representative of three independent experiments. Normal, n = 50 cell pairs; control MO, n = 50 cell pairs; ADAP MO, n = 50 cell pairs. Error bars represent ± SD. (Scale bars: 5 μm.)
Fig. 5.
MO-mediated ADAP knockdown abolishes synaptic dynein clustering but not β-catenin clustering in Jurkat (J) cells. Raji (R) cells were labeled with CTB, and both untreated (normal) and ADAP MO Jurkat–Raji cell pairs were prepared and immunostained as described. (a_–_d) Normal Jurkat–Raji pairs with Raji cells labeled with CTB were immunostained for ADAP (mouse anti-fyb, clone 5) (a), DIC (rabbit anti-DIC 1467) (b), and CTB (c); the red–green–blue overlay (d) shows typical ADAP and DIC clustering at the synapse. (e_–_h) Experiments parallel to those shown in a_–_d were carried out by using CTB-labeled Raji cells and ADAP-MO-treated Jurkat cells. Note that in the top row, there were four Jurkat cells bound to the central Raji cell. One of these cells still expressed ADAP after ADAP-MO electroporation and this cell shows dynein was also clustered at the synapse. The remaining cells show little or no ADAP or dynein at the synapse. (i_–_p) Normal (i_–_l) and ADAP MO (m_–_p) Jurkat–Raji pairs with Raji cells labeled with CTB were immunostained for ADAP (mouse anti-fyb, clone 5) (i and m) and β-catenin (rabbit anti-β-catenin) (j and n). The red–green–blue overlays (l and p) show typical β-catenin clustering at the synapse in both normal and ADAP MO Jurkat cells. Data are representative of two to three independent experiments. (Scale bars: 5 μm.)
Similar articles
- CLIP-170 is essential for MTOC repositioning during T cell activation by regulating dynein localisation on the cell surface.
Lim WM, Ito Y, Sakata-Sogawa K, Tokunaga M. Lim WM, et al. Sci Rep. 2018 Nov 28;8(1):17447. doi: 10.1038/s41598-018-35593-z. Sci Rep. 2018. PMID: 30487641 Free PMC article. - MTOC translocation modulates IS formation and controls sustained T cell signaling.
Martín-Cófreces NB, Robles-Valero J, Cabrero JR, Mittelbrunn M, Gordón-Alonso M, Sung CH, Alarcón B, Vázquez J, Sánchez-Madrid F. Martín-Cófreces NB, et al. J Cell Biol. 2008 Sep 8;182(5):951-62. doi: 10.1083/jcb.200801014. J Cell Biol. 2008. PMID: 18779373 Free PMC article. - The Rap1-cofilin-1 pathway coordinates actin reorganization and MTOC polarization at the B cell immune synapse.
Wang JC, Lee JY, Christian S, Dang-Lawson M, Pritchard C, Freeman SA, Gold MR. Wang JC, et al. J Cell Sci. 2017 Mar 15;130(6):1094-1109. doi: 10.1242/jcs.191858. Epub 2017 Feb 6. J Cell Sci. 2017. PMID: 28167682 - From lipid second messengers to molecular motors: microtubule-organizing center reorientation in T cells.
Huse M, Le Floc'h A, Liu X. Huse M, et al. Immunol Rev. 2013 Nov;256(1):95-106. doi: 10.1111/imr.12116. Immunol Rev. 2013. PMID: 24117815 Free PMC article. Review. - The role of the cytoskeleton at the immunological synapse.
Ritter AT, Angus KL, Griffiths GM. Ritter AT, et al. Immunol Rev. 2013 Nov;256(1):107-17. doi: 10.1111/imr.12117. Immunol Rev. 2013. PMID: 24117816 Free PMC article. Review.
Cited by
- Casein kinase I delta controls centrosome positioning during T cell activation.
Zyss D, Ebrahimi H, Gergely F. Zyss D, et al. J Cell Biol. 2011 Nov 28;195(5):781-97. doi: 10.1083/jcb.201106025. J Cell Biol. 2011. PMID: 22123863 Free PMC article. - HkRP3 is a microtubule-binding protein regulating lytic granule clustering and NK cell killing.
Ham H, Huynh W, Schoon RA, Vale RD, Billadeau DD. Ham H, et al. J Immunol. 2015 Apr 15;194(8):3984-96. doi: 10.4049/jimmunol.1402897. Epub 2015 Mar 11. J Immunol. 2015. PMID: 25762780 Free PMC article. - Formins regulate the actin-related protein 2/3 complex-independent polarization of the centrosome to the immunological synapse.
Gomez TS, Kumar K, Medeiros RB, Shimizu Y, Leibson PJ, Billadeau DD. Gomez TS, et al. Immunity. 2007 Feb;26(2):177-90. doi: 10.1016/j.immuni.2007.01.008. Immunity. 2007. PMID: 17306570 Free PMC article. - CLIP-170 is essential for MTOC repositioning during T cell activation by regulating dynein localisation on the cell surface.
Lim WM, Ito Y, Sakata-Sogawa K, Tokunaga M. Lim WM, et al. Sci Rep. 2018 Nov 28;8(1):17447. doi: 10.1038/s41598-018-35593-z. Sci Rep. 2018. PMID: 30487641 Free PMC article. - Cell polarity regulators, multifunctional organizers of lymphocyte activation and function.
Mastrogiovanni M, Di Bartolo V, Alcover A. Mastrogiovanni M, et al. Biomed J. 2022 Apr;45(2):299-309. doi: 10.1016/j.bj.2021.10.002. Epub 2021 Oct 7. Biomed J. 2022. PMID: 34626864 Free PMC article. Review.
References
- Poenie M, Kuhn JR, Combs J. Curr Opin Immunol. 2004;16:428–438. - PubMed
- Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Nature. 1998;395:82–86. - PubMed
- Kupfer A, Dennert G. J Immunol. 1984;133:2762–2766. - PubMed
- Kuhn JR, Poenie M. Immunity. 2002;16:111–121. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous