Aberrant Forkhead box O1 function is associated with impaired hepatic metabolism - PubMed (original) (raw)
Comparative Study
. 2006 Dec;147(12):5641-52.
doi: 10.1210/en.2006-0541. Epub 2006 Sep 21.
Affiliations
- PMID: 16997836
- PMCID: PMC2665253
- DOI: 10.1210/en.2006-0541
Comparative Study
Aberrant Forkhead box O1 function is associated with impaired hepatic metabolism
Shen Qu et al. Endocrinology. 2006 Dec.
Abstract
FoxO1 plays an important role in mediating the effect of insulin on hepatic metabolism. Increased FoxO1 activity is associated with reduced ability of insulin to regulate hepatic glucose production. However, the underlying mechanism and physiology remain unknown. We studied the effect of FoxO1 on the ability of insulin to regulate hepatic metabolism in normal vs. insulin-resistant liver under fed and fasting conditions. FoxO1 gain of function, as a result of adenovirus-mediated or transgenic expression, augmented hepatic gluconeogenesis, accompanied by decreased glycogen content and increased fat deposition in liver. Mice with excessive FoxO1 activity exhibited impaired glucose tolerance. Conversely, FoxO1 loss of function, caused by hepatic production of its dominant-negative variant, suppressed hepatic gluconeogenesis, resulting in enhanced glucose disposal and improved insulin sensitivity in db/db mice. FoxO1 expression becomes deregulated, culminating in increased nuclear localization and accounting for its increased transcription activity in livers of both high fat-induced obese mice and diabetic db/db mice. Increased FoxO1 activity resulted in up-regulation of hepatic peroxisome proliferator-activated receptor-gamma coactivator-1beta, fatty acid synthase, and acetyl CoA carboxylase expression, accounting for increased hepatic fat infiltration. These data indicate that hepatic FoxO1 deregulation impairs the ability of insulin to regulate hepatic metabolism, contributing to the development of hepatic steatosis and abnormal metabolism in diabetes.
Figures
Fig. 1
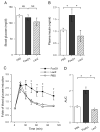
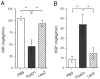
Effect of FoxO1 gain of function on glucose metabolism in normal mice. A, Fasting blood glucose levels. B, Fasting plasma insulin levels. C, Blood glucose profiles during GTT. D, AUC of blood glucose profiles during GTT. Blood glucose and plasma levels were determined after 16-h fasting on d 4 after vector administration. Data in C and D were obtained on d 5. *, P < 0.05 vs. controls. NS, Not significant.
Fig. 2
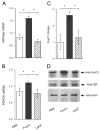
Effect of FoxO1 gain of function on hepatic gluconeogenic expression. Mice were killed after 16-h fasting 6 d after hepatic FoxO1 expression. Liver tissue was collected for preparation of total hepatic RNA. The relative hepatic mRNA levels of G6Pase (A) and PEPCK (B) were determined by real-time RT-PCR using _β_-actin mRNA as control. Hepatic nuclear proteins were subjected to immunoblot analysis using anti-FoxO1, anti-GK, and antiactin antibodies, respectively. The relative levels of hepatic FoxO1 protein were compared between FoxO1 vector- vs. control vector-treated mice (C). Representative immunoblots were shown in D. *, P < 0.05.
Fig. 3
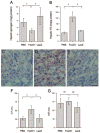
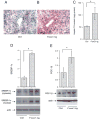
Hepatic glycogen and lipid content. Hepatic glycogen (A) and lipid (B) contents, defined as milligram of glycogen or triglyceride per gram of total liver protein, were determined as described in Materials and Methods. Sections of liver of mock- (C), FoxO1 vector- (D), and LacZ (E) vector-treated mice were examined histologically after Oil red O staining. Fasting plasma ALT (F) and AST (G) levels were determined at d 6 after vector administration. *, P < 0.01. Bar, 100 _μ_m.
Fig. 4
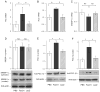
Impact of FoxO1 on hepatic lipogenic expression. Mice were killed after 16-h fasting after 6 d of hepatic FoxO1 production in normal CD-1 mice. Hepatic mRNA levels of FAS (A), ACC (B), and SREBP-1c (C) were determined using β_-actin mRNA as control. Hepatic protein levels of SREBP-1c (D) and PGC-1_β (E) were determined by semiquantitative immunoblot analysis. Cytosolic (molecular mass, 125 kDa) and nuclear (molecular mass, 68 kDa) SREBP-1 levels were coordinately produced. Quantitative data show hepatic levels of cytosolic SREBP-1c proteins. In addition, HepG2 cells were transduced with FoxO1 and LacZ vector at an MOI of 100 pfu/cell in 6-well plates. After 24 h incubation, PGC-1_β_ protein levels (F) were determined. *, P < 0.05. NS, Not significant.
Fig. 5
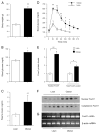
Hyperinsulinemic/euglycemic clamp studies. A, GIR. B, HGP rate. Three groups of CD-1 mice (n = 6, mean body weight, 33.7 ± 2.5 g) were mock treated or treated with FoxO1 or LacZ vector. Animals were cannulated on d 3 after treatment. After 1 d of recovery, animals were subjected to hyperinsulinemic/euglycemic clamping for the determination of GIR and HGP as described in Materials and Methods. *, P < 0.01.
Fig. 6
Effect of FoxO1 on hepatic metabolism in FoxO1S253A transgenic mice. FoxO1S253A transgenic mice (Foxo1-tg, n = 5) at 8 months of age and control littermates (Ctrl, n = 7) were killed after 16-h fasting. Liver tissues were studied for hepatic fat content by staining with Oil red O (A and B) and quantifying hepatic TG content (C). Hepatic protein levels of SREBP-1c (D) and PGC-1_β_ (E) were quantified by a semiquantitative immunoblot assay. Quantitative data show hepatic abundance of cytosolic SREBP-1c proteins because cytosolic and nuclear SREBP-1 levels were coordinately expressed. *, P < 0.05. Bar, 50 _μ_m.
Fig. 7
FoxO1 expression in high-fat diet-induced obese mice. C57 BL/6J mice at 6 wk of age were fed a high-fat diet (n = 6) or regular chow (n = 6) for 2 months. A, Body weight. B, Fasting blood glucose levels. C, Fasting plasma insulin levels. D, Blood glucose profiles during GTT. E, Nuclear and total FoxO1 protein levels. F, Immunoblot of cytoplasmic vs. nuclear FoxO1 protein. G, FoxO1 mRNA levels. Total liver RNA was prepared for the determination of FoxO1 mRNA levels by semiquantitative RT-PCR using _β_-actin mRNA as control. Fasting blood glucose and plasma insulin levels were determined after a 16-h fast. *, P < 0.05; **, P < 0.001.
Fig. 8
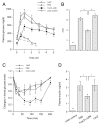
Effect of FoxO1 loss of function on glucose metabolism in db/db mice. A, Blood glucose profiles during GTT. B, AUC of blood glucose profiles during GTT. C, Changes in blood glucose levels in response to insulin tolerance test. D, Fasting plasma insulin levels. Data in A and B were obtained on d 4 after vector administration. Insulin tolerance test was performed on d 5 after vector administration. For blood glucose levels higher than 500 mg/dl, aliquots of 5-_μ_l freshly collected tail-vein blood samples were mixed with 5 _μ_l PBS buffer before blood glucose levels were immediately measured by glucometer. *, P < 0.05; **, P < 0.001.
Fig. 9
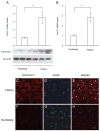
Hepatic FoxO1 expression in fed and fasting states. Male C57BL/6J mice (6 months old on regular chow) were fasted for 24 h (n = 3) or fed ad libitum (n = 4). Liver tissue was collected from killed mice for the preparation of liver protein extracts. Aliquots of 20 _μ_g liver proteins were subjected to 4–20% gradient SDS-PAGE. Hepatic abundance of FoxO1 (A) was determined by semiquantitative immunoblot assay. Hepatic FoxO1 mRNA levels (B) were determined by real-time RT-PCR using _β_-actin mRNA as control. Hepatic FoxO1 subcellular distribution in fasting (C–E) and nonfasting (F–H) states was examined by immunohistochemistry using anti-FoxO1 antibody. Arrows mark FoxO1-colocalized nuclei (E). Bar, 20 _μ_m. *, P < 0.001.
Similar articles
- FoxO1 integrates insulin signaling to VLDL production.
Kamagate A, Dong HH. Kamagate A, et al. Cell Cycle. 2008 Oct;7(20):3162-70. doi: 10.4161/cc.7.20.6882. Epub 2008 Oct 27. Cell Cycle. 2008. PMID: 18927507 Free PMC article. Review. - FoxO1 links hepatic insulin action to endoplasmic reticulum stress.
Kamagate A, Kim DH, Zhang T, Slusher S, Gramignoli R, Strom SC, Bertera S, Ringquist S, Dong HH. Kamagate A, et al. Endocrinology. 2010 Aug;151(8):3521-35. doi: 10.1210/en.2009-1306. Epub 2010 May 25. Endocrinology. 2010. PMID: 20501674 Free PMC article. - Inhibition of Foxo1 function is associated with improved fasting glycemia in diabetic mice.
Altomonte J, Richter A, Harbaran S, Suriawinata J, Nakae J, Thung SN, Meseck M, Accili D, Dong H. Altomonte J, et al. Am J Physiol Endocrinol Metab. 2003 Oct;285(4):E718-28. doi: 10.1152/ajpendo.00156.2003. Epub 2003 Jun 3. Am J Physiol Endocrinol Metab. 2003. PMID: 12783775 - Regulation of glucose metabolism via hepatic forkhead transcription factor 1 (FoxO1) by Morinda citrifolia (noni) in high-fat diet-induced obese mice.
Nerurkar PV, Nishioka A, Eck PO, Johns LM, Volper E, Nerurkar VR. Nerurkar PV, et al. Br J Nutr. 2012 Jul;108(2):218-228. doi: 10.1017/S0007114511005563. Epub 2011 Oct 20. Br J Nutr. 2012. PMID: 22011624 Free PMC article. - FoxO1, the transcriptional chief of staff of energy metabolism.
Kousteni S. Kousteni S. Bone. 2012 Feb;50(2):437-43. doi: 10.1016/j.bone.2011.06.034. Epub 2011 Jul 28. Bone. 2012. PMID: 21816244 Free PMC article. Review.
Cited by
- FOXO1 mediates the autocrine effect of endothelin-1 on endothelial cell survival.
Cifarelli V, Lee S, Kim DH, Zhang T, Kamagate A, Slusher S, Bertera S, Luppi P, Trucco M, Dong HH. Cifarelli V, et al. Mol Endocrinol. 2012 Jul;26(7):1213-24. doi: 10.1210/me.2011-1276. Epub 2012 May 8. Mol Endocrinol. 2012. PMID: 22570335 Free PMC article. - Molecular pathways in non-alcoholic fatty liver disease.
Berlanga A, Guiu-Jurado E, Porras JA, Auguet T. Berlanga A, et al. Clin Exp Gastroenterol. 2014 Jul 5;7:221-39. doi: 10.2147/CEG.S62831. eCollection 2014. Clin Exp Gastroenterol. 2014. PMID: 25045276 Free PMC article. Review. - FoxO1 integrates insulin signaling to VLDL production.
Kamagate A, Dong HH. Kamagate A, et al. Cell Cycle. 2008 Oct;7(20):3162-70. doi: 10.4161/cc.7.20.6882. Epub 2008 Oct 27. Cell Cycle. 2008. PMID: 18927507 Free PMC article. Review. - Wnt signaling regulates hepatic metabolism.
Liu H, Fergusson MM, Wu JJ, Rovira II, Liu J, Gavrilova O, Lu T, Bao J, Han D, Sack MN, Finkel T. Liu H, et al. Sci Signal. 2011 Feb 1;4(158):ra6. doi: 10.1126/scisignal.2001249. Sci Signal. 2011. PMID: 21285411 Free PMC article. - Mechanism of Lipid Accumulation through PAR2 Signaling in Diabetic Male Mice.
Kim DH, Kim YR, Bang E, Ha S, Noh SG, Kim BM, Jeong SH, Jung HJ, Lee JY, Chung HY. Kim DH, et al. Endocrinol Metab (Seoul). 2021 Feb;36(1):171-184. doi: 10.3803/EnM.2020.850. Epub 2021 Feb 24. Endocrinol Metab (Seoul). 2021. PMID: 33677938 Free PMC article.
References
- Pilkis SJ, Granner DK. Molecular physiology of the regulation of hepatic gluconeogenesis and glycogenolysis. Annu Rev Physiol. 1992;54:885–909. - PubMed
- Giaccari A, Morviducci L, Pastore L, Zorretta D, Sbraccia P, Maroccia E, Buongiorno A, Tamburrano G. Relative contribution of glycogenolysis and gluconeogenesis to hepatic glucose production in control and diabetic rats. A re-examination in the presence of euglyceamia. Diabetologia. 1998;41:307–314. - PubMed
- Boden G, Chen X, Stein TP. Gluconeogenesis in moderately and severely hyperglycemic patients with type 2 diabetes mellitus. Am J Physiol Endocrinol Metab. 2001;280:E23–E30. - PubMed
- Kaestner KH, Knochel W, Martinez DE. Unified nomenclature for the winged helix/forkhead transcription factors. Gene Dev. 2000;14:142–146. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous