A conserved supergene locus controls colour pattern diversity in Heliconius butterflies - PubMed (original) (raw)
doi: 10.1371/journal.pbio.0040303.
Riccardo Papa, Margarita Beltrán, Nicola Chamberlain, Jesús Mavárez, Simon Baxter, Moisés Abanto, Eldredge Bermingham, Sean J Humphray, Jane Rogers, Helen Beasley, Karen Barlow, Richard H ffrench-Constant, James Mallet, W Owen McMillan, Chris D Jiggins
Affiliations
- PMID: 17002517
- PMCID: PMC1570757
- DOI: 10.1371/journal.pbio.0040303
A conserved supergene locus controls colour pattern diversity in Heliconius butterflies
Mathieu Joron et al. PLoS Biol. 2006 Oct.
Abstract
We studied whether similar developmental genetic mechanisms are involved in both convergent and divergent evolution. Mimetic insects are known for their diversity of patterns as well as their remarkable evolutionary convergence, and they have played an important role in controversies over the respective roles of selection and constraints in adaptive evolution. Here we contrast three butterfly species, all classic examples of Müllerian mimicry. We used a genetic linkage map to show that a locus, Yb, which controls the presence of a yellow band in geographic races of Heliconius melpomene, maps precisely to the same location as the locus Cr, which has very similar phenotypic effects in its co-mimic H. erato. Furthermore, the same genomic location acts as a "supergene", determining multiple sympatric morphs in a third species, H. numata. H. numata is a species with a very different phenotypic appearance, whose many forms mimic different unrelated ithomiine butterflies in the genus Melinaea. Other unlinked colour pattern loci map to a homologous linkage group in the co-mimics H. melpomene and H. erato, but they are not involved in mimetic polymorphism in H. numata. Hence, a single region from the multilocus colour pattern architecture of H. melpomene and H. erato appears to have gained control of the entire wing-pattern variability in H. numata, presumably as a result of selection for mimetic "supergene" polymorphism without intermediates. Although we cannot at this stage confirm the homology of the loci segregating in the three species, our results imply that a conserved yet relatively unconstrained mechanism underlying pattern switching can affect mimicry in radically different ways. We also show that adaptive evolution, both convergent and diversifying, can occur by the repeated involvement of the same genomic regions.
Conflict of interest statement
Competing interests. The authors have declared that no competing interests exist.
Figures
Figure 1. Colour Pattern Diversity of H. numata, H. melpomene, and their Respective Co-Mimics
The upper half of the figure shows five sympatric forms of H. numata from northern Peru (second row, left to right: H. n. f. tarapotensis, H. n. f. silvana, H. n. f. aurora, H. n. f. bicoloratus, and H. n. f. arcuella) with their distantly related comimetic Melinaea species (Nymphalidae: Ithomiinae) from the same area (first row: M. menophilus ssp. nov_._, M. ludovica ludovica, M. marsaeus rileyi, M. marsaeus mothone, and M. marsaeus phasiana) [20]. The lower half of the figure shows five colour pattern races of H. melpomene, each from a different area of South America (third row: H. m. rosina, H. m. cythera, H. m. aglaope, H. m. melpomene, and H. m. plesseni) with their distantly related comimetic H. erato races from the same areas (fourth row: H. e. cf. petiveranus, H. e. cyrbia, H. e. emma, H. e. hydara, and H. e. notabilis). H. m. aglaope and H. e. emma are known as rayed forms, whereas H. m. rosina, H. m. melpomene, and co-mimics are known as postman forms. H. melpomene and H. erato are from divergent clades of Heliconius and are identified in the field using minor morphological characters, such as the different form of the red rays on the hindwing between H. m. aglaope and H. e. emma (third from left) or the arrangement of red versus white patches in H. m. plesseni and H. e. notabilis (first from right). Co-mimics H. numata and Melinaea spp. belong to different subfamilies of the Nymphalidae and have very different body morphology and wing venation. The phylogram on the left is a maximum-likelihood tree based on 1,541 bases of mitochondrial DNA (scale bar in substitutions per site, all bootstrap values over 99).
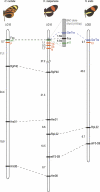
Figure 2. Crosses Used for Mapping the Yb, P, and Cr Loci
(A) Crossing scheme in H. melpomene showing segregation of tightly linked loci Yb and Sb (hindwing yellow bar and white margin, present in H. m. cythera, YbcYbc SbcSbc) in brood B033. Genotypes are shown on the figure_._ The hindwing image in the box has been manipulated to highlight the shadow hindwing bar characteristic of heterozygote genotypes. Segregation of the linked N locus controlling the yellow forewing band was followed in a different set of crosses not shown here (Table S1; Materials and Methods). (B) Crossing scheme in H. erato showing segregation of Cr alleles in brood CH-CH5; Cr controls the forewing yellow band (absent in H. e. cyrbia, CrcCrc), and the hindwing yellow bar and white margin (present in H. e. cyrbia). The red-patterning gene D also segregates in this cross, but is unlinked to Cr; only progeny with a DhiDhi genotype are shown on the figure (Table S2; see also [24] for a figure of a similar cross showing all nine possible genotypes). (C) Crossing scheme in H. numata showing segregation of the P alleles in intercross B502. F1 parents are heterozygous for different alleles, thus producing four different genotypes in the progeny. P switches the entire colour pattern, with strong dominance between sympatric alleles. Other broods (not shown) segregating for the very same Pele and Psil alleles were sired by the same male or its full brother (Table S3).
Figure 3. Chromosomal Maps for Linkage Group Homologues in H. melpomene (LG15), H. numata (LG15), and H. erato (LG02)
Distances are in Haldane centimorgans. The alternative orders for P and a41 relative to Hm01 in H. numata are not significantly different (ΔLn_L_ = −1.40). Similarly, most orders of N, Sb, Yb, and a41 in H. melpomene are not significantly different (from ΔLn_L_ = −0.15 for the order a41–Yb_–_N–Sb– to ΔLn_L_ = −0.77 for a41–N–Yb–Sb–). Finally, the two orders for Cr and GerTra in H. erato are also equally significant. Therefore, we here show the most likely gene orders but cannot exclude that the colour loci are on the other side of the anchor loci a41, Fox, or GerTra. In contrast, anchor loci order GerTra–RpP40–Hm01–Hm08 is robust, with alternative orders significantly worse (ΔLn_L_ < −2), although the relative placement of _RpL22_ and _eIF3_-_S9_ is uncertain in H. melpomene and H. erato (ΔLn_L_ > −2).
Figure 4. Annotation of Clone AEHM-41C10 from the Heliconius melpomene BAC Library
The region is situated on LG15 in the H. melpomene genome [22]. The sequence contains open reading frames of strong homology to 12 reported genes, three of which appear to be retrotransposon-associated coding regions (dotted boxes). Also highlighted in double frames are the a41 marker, which was used in H. numata and H. melpomene crosses and to isolate the clone from the library, and the Rabgeranylgeranyl transferase gene, used as a marker in H. erato crosses.
Comment in
- Repeating patterns of mimicry.
Meyer A. Meyer A. PLoS Biol. 2006 Oct;4(10):e341. doi: 10.1371/journal.pbio.0040341. PLoS Biol. 2006. PMID: 17048984 Free PMC article. - Jack-of-all-trades "supergene" controls butterfly wing pattern diversity.
Gross L. Gross L. PLoS Biol. 2006 Oct;4(10):e329. doi: 10.1371/journal.pbio.0040329. Epub 2006 Sep 26. PLoS Biol. 2006. PMID: 20076469 Free PMC article. No abstract available.
Similar articles
- Convergent evolution in the genetic basis of Müllerian mimicry in heliconius butterflies.
Baxter SW, Papa R, Chamberlain N, Humphray SJ, Joron M, Morrison C, ffrench-Constant RH, McMillan WO, Jiggins CD. Baxter SW, et al. Genetics. 2008 Nov;180(3):1567-77. doi: 10.1534/genetics.107.082982. Epub 2008 Sep 14. Genetics. 2008. PMID: 18791259 Free PMC article. - Highly conserved gene order and numerous novel repetitive elements in genomic regions linked to wing pattern variation in Heliconius butterflies.
Papa R, Morrison CM, Walters JR, Counterman BA, Chen R, Halder G, Ferguson L, Chamberlain N, Ffrench-Constant R, Kapan DD, Jiggins CD, Reed RD, McMillan WO. Papa R, et al. BMC Genomics. 2008 Jul 22;9:345. doi: 10.1186/1471-2164-9-345. BMC Genomics. 2008. PMID: 18647405 Free PMC article. - _Cortex cis_-regulatory switches establish scale colour identity and pattern diversity in Heliconius.
Livraghi L, Hanly JJ, Van Bellghem SM, Montejo-Kovacevich G, van der Heijden ES, Loh LS, Ren A, Warren IA, Lewis JJ, Concha C, Hebberecht L, Wright CJ, Walker JM, Foley J, Goldberg ZH, Arenas-Castro H, Salazar C, Perry MW, Papa R, Martin A, McMillan WO, Jiggins CD. Livraghi L, et al. Elife. 2021 Jul 19;10:e68549. doi: 10.7554/eLife.68549. Elife. 2021. PMID: 34280087 Free PMC article. - Heliconius wing patterns: an evo-devo model for understanding phenotypic diversity.
Joron M, Jiggins CD, Papanicolaou A, McMillan WO. Joron M, et al. Heredity (Edinb). 2006 Sep;97(3):157-67. doi: 10.1038/sj.hdy.6800873. Epub 2006 Jul 12. Heredity (Edinb). 2006. PMID: 16835591 Review. - The functional basis of wing patterning in Heliconius butterflies: the molecules behind mimicry.
Kronforst MR, Papa R. Kronforst MR, et al. Genetics. 2015 May;200(1):1-19. doi: 10.1534/genetics.114.172387. Genetics. 2015. PMID: 25953905 Free PMC article. Review.
Cited by
- Supergene evolution via gain of autoregulation.
VanKuren NW, Sheikh SI, Fu CL, Massardo D, Lu W, Kronforst MR. VanKuren NW, et al. bioRxiv [Preprint]. 2024 Dec 16:2024.01.09.574839. doi: 10.1101/2024.01.09.574839. bioRxiv. 2024. PMID: 38260248 Free PMC article. Preprint. - Stripes, sex and sparrows: what processes underlie heteromorphic chromosome evolution?
Joron M, Whibley A. Joron M, et al. Heredity (Edinb). 2011 Apr;106(4):531-2. doi: 10.1038/hdy.2010.106. Epub 2010 Aug 18. Heredity (Edinb). 2011. PMID: 20717159 Free PMC article. No abstract available. - Does coevolution in refugia drive mimicry in bumble bees? Insights from a South Asian mimicry group.
Cui J, Chen Y, Hines HM, Ma L, Yang W, Wang C, Liu S, Li H, Cai W, Da W, Williams P, Tian L. Cui J, et al. Sci Adv. 2024 Jun 14;10(24):eadl2286. doi: 10.1126/sciadv.adl2286. Epub 2024 Jun 12. Sci Adv. 2024. PMID: 38865449 Free PMC article. - Moving Speciation Genetics Forward: Modern Techniques Build on Foundational Studies in Drosophila.
Castillo DM, Barbash DA. Castillo DM, et al. Genetics. 2017 Nov;207(3):825-842. doi: 10.1534/genetics.116.187120. Genetics. 2017. PMID: 29097397 Free PMC article. Review. - Convergent, modular expression of ebony and tan in the mimetic wing patterns of Heliconius butterflies.
Ferguson LC, Maroja L, Jiggins CD. Ferguson LC, et al. Dev Genes Evol. 2011 Dec;221(5-6):297-308. doi: 10.1007/s00427-011-0380-6. Epub 2011 Dec 3. Dev Genes Evol. 2011. PMID: 22139062
References
- Carroll SB, Grenier JK, Weatherbee SD. From DNA to diversity: Molecular genetics and the evolution of animal design. Malden (Massachusetts): Blackwell Science; 2001. 214. p.
- Gompel N, Prud'homme B, Wittkopp PJ, Kassner VA, Carroll SB. Chance caught on the wing: cis-regulatory evolution and the origin of pigment patterns in Drosophila . Nature. 2005;433:481–487. - PubMed
- Müller F. Ituna and Thyridia: A remarkable case of mimicry in butterflies. Trans Entomol Soc Lond 1879. 1879. pp. xx–xxix.
- Fisher RA. The genetical theory of natural selection, 2nd revised edition. New York: Dover; 1958. 291. p.
- Goldschmidt RB. Mimetic polymorphism, a controversial chapter of Darwinism. Q Rev Biol. 1945;20:147–164. 205–230.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources