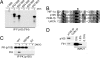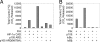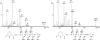Posttranslational hydroxylation of ankyrin repeats in IkappaB proteins by the hypoxia-inducible factor (HIF) asparaginyl hydroxylase, factor inhibiting HIF (FIH) - PubMed (original) (raw)
. 2006 Oct 3;103(40):14767-72.
doi: 10.1073/pnas.0606877103. Epub 2006 Sep 26.
David E Lancaster, Ineke P Stolze, Kirsty S Hewitson, Michael A McDonough, Mathew L Coleman, Charlotte H Coles, Xiaohong Yu, Ronald T Hay, Steven C Ley, Christopher W Pugh, Neil J Oldham, Norma Masson, Christopher J Schofield, Peter J Ratcliffe
Affiliations
- PMID: 17003112
- PMCID: PMC1578504
- DOI: 10.1073/pnas.0606877103
Posttranslational hydroxylation of ankyrin repeats in IkappaB proteins by the hypoxia-inducible factor (HIF) asparaginyl hydroxylase, factor inhibiting HIF (FIH)
Matthew E Cockman et al. Proc Natl Acad Sci U S A. 2006.
Abstract
Studies on hypoxia-sensitive pathways have revealed a series of Fe(II)-dependent dioxygenases that regulate hypoxia-inducible factor (HIF) by prolyl and asparaginyl hydroxylation. The recognition of these unprecedented signaling processes has led to a search for other substrates of the HIF hydroxylases. Here we show that the human HIF asparaginyl hydroxylase, factor inhibiting HIF (FIH), also efficiently hydroxylates specific asparaginyl (Asn)-residues within proteins of the IkappaB family. After the identification of a series of ankyrin repeat domain (ARD)-containing proteins in a screen for proteins interacting with FIH, the ARDs of p105 (NFKB1) and IkappaBalpha were shown to be efficiently hydroxylated by FIH at specific Asn residues in the hairpin loops linking particular ankyrin repeats. The target Asn residue is highly conserved as part of the ankyrin consensus, and peptides derived from a diverse range of ARD-containing proteins supported FIH enzyme activity. These findings demonstrate that this type of protein hydroxylation is not restricted to HIF and strongly suggest that FIH-dependent ARD hydroxylation is a common occurrence, potentially providing an oxygen-sensitive signal to a diverse range of processes.
Figures
Fig. 1.
Interactions between p105 and FIH. (A) Interaction of [35S]methionine-labeled p105 polypeptides with extract from control cells (−) or cells induced with doxycycline (DOX) to express FLAG-FIH (+). Anti-FLAG immunoprecipitations; coprecipitating products are visualized by autoradiography. Residues 650–684 are essential for FIH interaction. (B) Alignment of residues 667–690 (fourth ankyrin repeat) of p105 with the ARD of FEM1β and UACA as well as the HIF-1α hydroxylation site (∗). (C) Interaction between FIH and transfected full-length PK-tagged p105, Asn-678 mutant p105 (N678A), in 293T cells exposed to DMOG (+) or vehicle control (−). EV, empty vector control. Anti-PK immunoprecipitates; coprecipitating FIH is detected by anti-FIH mAb. (D) Coimmunoprecipitation of endogenous FIH with p105 from HeLa cell extract. p105 IP, antibody to the C terminus of p105; CON IP, preimmune serum.
Fig. 2.
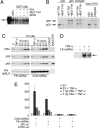
Recombinant p105 and IκBα promote decarboxylation of 2-OG in assays of FIH activity. 2-OG decarboxylation is measured by 14CO2 formation. (A) Reactions containing the indicated GST-tagged proteins: HIF-1α 775–826 (HIF-1α C-terminal activation domain); p105 ARD 537–809 (p105 ARD); p105 ARD Asn-678 mutant (p105 ARD(N678A)). Wild type (p105 ARD) but not the N678A mutant promoted 2-OG decarboxylation. Higher background was observed with p105 ARD(N678A) because of less pure preparation of protein. (B) Experiments comparing p105 ARD with full-length IκBα and IκBβ proteins. IκBα, but not IκBβ, promotes 2-OG decarboxylation.
Fig. 3.
Mass spectrometric analysis of FIH-mediated hydroxylation of p105 and IκBα in vivo. (A) LC/MS spectrum from a tryptic digest of PK-tagged p105 immunopurified from transfected 293T cells. Peaks at m/z = 763.87 and 771.87 correspond to [M + 2H]2+ of unhydroxylated and hydroxylated LLVAAGADVNAQEQK peptides, respectively. Mutations (L668K/R870A) were introduced into p105 to enable LC/MS and MS/MS analysis of tryptic peptides. (B) MS/MS spectra of the m/z 763.87 (Left) and m/z 771.87 (Right) parent ions assign Asn-678 as the site of hydroxylation in p105. A +16-Da shift appears in the y-ion series appearing at y6, corresponding to fragments containing Asn-678. (C) Extracted ion chromatograms illustrating the effect of FIH intervention on IκBα hydroxylation at Asn-244. (Left) Standards for the hydroxylated and unhydroxylated modified tryptic peptides (see Fig. 9 for MS/MS assignments). (Right) Tryptic digest of HA-tagged IκBα immunopurified from transfected 293T cells; FIH overexpression increases, whereas FIH siRNA decreases, the ratio of hydroxylated to unhydroxylated peptide. (∗C indicates derivatization on cysteine; see Experimental Procedures.)
Fig. 4.
Effects of FIH on IκB/NF-κB complex formation and activity. (A) EMSA of NF-κB DNA-binding activity in nuclear extracts of TNF-α-stimulated HeLa cells. Effect of recombinant IκBα, GST-IκBα, and GST-IκBα incubated in vitro with FIH, FIH plus inhibitor (_N_-oxalylglycine, NOG), or mutant D201A FIH (mut FIH). Slightly more potent inhibitory effects of hydroxylated IκBα (lane 4) were consistently observed. Arrow indicates the NF-κB DNA-binding activity. IκBα inputs were normalized but not shown. (B) Interaction of recombinant GST-IκBα, pretreated with either wild type or mutant D201A FIH (mut FIH), with 35S-labeled p50 and/or p65 produced by IVTT in wheat germ lysate. Interaction was performed in the presence of BSA, assayed by GST pull-down. Coprecipitating p50 and p65 are detected by autoradiography. GST-IκBα input is normalized but not shown. (C) Association of IκBα with p65 and p50 in cells. U2OS cells were transfected with control or FIH siRNA and stimulated with TNF-α. Anti-IκBα immunoprecipitates were immunoblotted with anti-IκBα, anti-p50, or anti-p65 antibodies. Cells were harvested either at time 0 (untreated), after a 30-min exposure to TNF-α (TNF-α), or at the indicated times (in minutes) after removal of TNF-α. (D) NF-κB DNA-binding activity. EMSA of nuclear extracts from serum-deprived 293T cells transfected with control (−) or FIH-specific (+) siRNA duplexes and treated with TNF-α for 30 min. Arrow indicates the NF-κB DNA-binding activity. (E) NF-κB reporter activity. 293T cells were treated with FIH or control siRNA then transfected with NF-κB (κB LUC) or control (CON LUC) reporter plasmids. Cells were stimulated with TNF-α for 6 h to induce NF-κB activity, and the ability of cotransfected IκBα to suppress reporter gene activity was assessed. Relative luciferase activity is shown as the mean ± 1 SD of triplicate samples from a representative experiment.
Fig. 5.
MS demonstrating hydroxylation of endogenous IκBα. MS/MS spectra are depicted showing the N-terminally modified *CGADVNR peptide, derived by 6-exo-trig cyclization from the peptide _S_-alkylated with iodoacetamide. (Left) Fragments of the unhydroxylated IκBα peptide, [M+H]+ = m/z 774.3. (Right) Fragments of the hydroxylated IκBα peptide, [M+H]+ = m/z 790.3; a +16-Da shift is observed in the y-ion series, appearing at the y2 ion, which corresponds to fragments containing Asn-244.
Similar articles
- Proteomics-based identification of novel factor inhibiting hypoxia-inducible factor (FIH) substrates indicates widespread asparaginyl hydroxylation of ankyrin repeat domain-containing proteins.
Cockman ME, Webb JD, Kramer HB, Kessler BM, Ratcliffe PJ. Cockman ME, et al. Mol Cell Proteomics. 2009 Mar;8(3):535-46. doi: 10.1074/mcp.M800340-MCP200. Epub 2008 Oct 20. Mol Cell Proteomics. 2009. PMID: 18936059 Free PMC article. - Asparaginyl hydroxylation of the Notch ankyrin repeat domain by factor inhibiting hypoxia-inducible factor.
Coleman ML, McDonough MA, Hewitson KS, Coles C, Mecinovic J, Edelmann M, Cook KM, Cockman ME, Lancaster DE, Kessler BM, Oldham NJ, Ratcliffe PJ, Schofield CJ. Coleman ML, et al. J Biol Chem. 2007 Aug 17;282(33):24027-38. doi: 10.1074/jbc.M704102200. Epub 2007 Jun 15. J Biol Chem. 2007. PMID: 17573339 - FIH-dependent asparaginyl hydroxylation of ankyrin repeat domain-containing proteins.
Cockman ME, Webb JD, Ratcliffe PJ. Cockman ME, et al. Ann N Y Acad Sci. 2009 Oct;1177:9-18. doi: 10.1111/j.1749-6632.2009.05042.x. Ann N Y Acad Sci. 2009. PMID: 19845602 Review. - Ankyrin Repeat Proteins of Orf Virus Influence the Cellular Hypoxia Response Pathway.
Chen DY, Fabrizio JA, Wilkins SE, Dave KA, Gorman JJ, Gleadle JM, Fleming SB, Peet DJ, Mercer AA. Chen DY, et al. J Virol. 2016 Dec 16;91(1):e01430-16. doi: 10.1128/JVI.01430-16. Print 2017 Jan 1. J Virol. 2016. PMID: 27795413 Free PMC article. - Signalling cross talk of the HIF system: involvement of the FIH protein.
Coleman ML, Ratcliffe PJ. Coleman ML, et al. Curr Pharm Des. 2009;15(33):3904-7. doi: 10.2174/138161209789649448. Curr Pharm Des. 2009. PMID: 19671041 Review.
Cited by
- Redox control of inflammation in macrophages.
Brüne B, Dehne N, Grossmann N, Jung M, Namgaladze D, Schmid T, von Knethen A, Weigert A. Brüne B, et al. Antioxid Redox Signal. 2013 Aug 20;19(6):595-637. doi: 10.1089/ars.2012.4785. Epub 2013 Mar 6. Antioxid Redox Signal. 2013. PMID: 23311665 Free PMC article. Review. - Role of Hypoxia in the Control of the Cell Cycle.
Druker J, Wilson JW, Child F, Shakir D, Fasanya T, Rocha S. Druker J, et al. Int J Mol Sci. 2021 May 5;22(9):4874. doi: 10.3390/ijms22094874. Int J Mol Sci. 2021. PMID: 34062959 Free PMC article. Review. - The asparaginyl hydroxylase factor inhibiting HIF-1alpha is an essential regulator of metabolism.
Zhang N, Fu Z, Linke S, Chicher J, Gorman JJ, Visk D, Haddad GG, Poellinger L, Peet DJ, Powell F, Johnson RS. Zhang N, et al. Cell Metab. 2010 May 5;11(5):364-78. doi: 10.1016/j.cmet.2010.03.001. Epub 2010 Apr 15. Cell Metab. 2010. PMID: 20399150 Free PMC article. - Mint3 enhances the activity of hypoxia-inducible factor-1 (HIF-1) in macrophages by suppressing the activity of factor inhibiting HIF-1.
Sakamoto T, Seiki M. Sakamoto T, et al. J Biol Chem. 2009 Oct 30;284(44):30350-9. doi: 10.1074/jbc.M109.019216. Epub 2009 Sep 2. J Biol Chem. 2009. PMID: 19726677 Free PMC article. - Mint3 as a Potential Target for Cooling Down HIF-1α-Mediated Inflammation and Cancer Aggressiveness.
Tanaka N, Sakamoto T. Tanaka N, et al. Biomedicines. 2023 Feb 14;11(2):549. doi: 10.3390/biomedicines11020549. Biomedicines. 2023. PMID: 36831085 Free PMC article. Review.
References
- Semenza GL. Annu Rev Cell Dev Biol. 1999;15:551–578. - PubMed
- Kaelin WG., Jr Annu Rev Biochem. 2004;74:115–128. - PubMed
- Schofield CJ, Ratcliffe PJ. Nat Rev Mol Cell Biol. 2004;5:343–354. - PubMed
- Baek JH, Mahon PC, Oh J, Kelly B, Krishnamachary B, Pearson M, Chan DA, Giaccia AJ, Semenza GL. Mol Cell. 2005;17:503–512. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- WT_/Wellcome Trust/United Kingdom
- B18672/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/B/07683/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- G116/127/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous