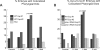Temporal regulation of foregut development by HTZ-1/H2A.Z and PHA-4/FoxA - PubMed (original) (raw)
Temporal regulation of foregut development by HTZ-1/H2A.Z and PHA-4/FoxA
Dustin L Updike et al. PLoS Genet. 2006.
Abstract
The histone variant H2A.Z is evolutionarily conserved and plays an essential role in mice, Drosophila, and Tetrahymena. The essential function of H2A.Z is unknown, with some studies suggesting a role in transcriptional repression and others in activation. Here we show that Caenorhabditis elegans HTZ-1/H2A.Z and the remodeling complex MYS-1/ESA1-SSL-1/SWR1 synergize with the FoxA transcription factor PHA-4 to coordinate temporal gene expression during foregut development. We observe dramatic genetic interactions between pha-4 and htz-1, mys-1, and ssl-1. A survey of transcription factors reveals that this interaction is specific, and thus pha-4 is acutely sensitive to reductions in these three proteins. Using a nuclear spot assay to visualize HTZ-1 in living embryos as organogenesis proceeds, we show that HTZ-1 is recruited to foregut promoters at the time of transcriptional onset, and this recruitment requires PHA-4. Loss of htz-1 by RNAi is lethal and leads to delayed expression of a subset of foregut genes. Thus, the effects of PHA-4 on temporal regulation can be explained in part by recruitment of HTZ-1 to target promoters. We suggest PHA-4 and HTZ-1 coordinate temporal gene expression by modulating the chromatin environment.
Conflict of interest statement
Competing interests. The authors have declared that no competing interests exist.
Figures
Figure 1. Enhancement of pha-4(ts) by mys-1, ssl-1, and htz-1
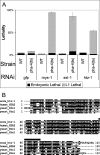
(A) Feeding dsRNA to wild-type (WT) or pha-4(ts) [18,25] worms at the permissive temperature of 24 °C. WT worms generate viable progeny with mys-1, ssl-1 or htz-1 RNAi. In the pha-4(ts) background, L1 arrest increased with mys-1, ssl-1, or htz-1 RNAi compared to control GFP(RNAi) (grey bars). Embryonic lethality remained unchanged (black bars). Effectiveness of RNAi feeding was manifest through viable, but sterile, progeny for mys-1 and ssl-1 [14], as well as repeated enhancement of L1 lethality for pha-4(ts), performed in parallel. n = 100 worms/plate, three plates per column. Error bars indicate the standard deviation. (B) Alignment of C. elegans htz-1 (R08C7.3) with human H2A.Z, yeast Htz1, and one of the core H2A genes from yeast, Hta1. Extended acid patch region essential for H2A.Z function is indicated by the bar [50,60,67].
Figure 2. mys-1, ssl-1, and htz-1 Enhance Pharyngeal Defects of pha-4(ts)
(A–C) Feeding dsRNA to pha-4(ts) worms at the permissive temperature of 24 °C. (A) A pha-4(ts); GFP(RNAi) L1 with a wild-type pharynx (arrowheads). (B) A pha-4(ts); ssl-1(RNAi) L1 with an unattached pharynx (arrowheads). (C) Quantitation of pha-4(ts) animals exhibiting a normal pharynx (WT), an unattached or incomplete pharynx (Pun), or no detectable pharynx. htz-1, ssl-1, or mys-1 RNAi significantly increased the number of Pun pha-4(ts) animals (_htz-1: p_=0.0290; ssl-1 and mys-1: p < 0.0001, Fisher exact test). (D–F) Feeding dsRNA to pha-4(ts) animals at the intermediate temperature of 20 °C. (D) A pha-4(ts); GFP(RNAi) L1 at the intermediate temperature of 20 °C with a morphologically wild-type pharynx (arrowheads). (E) A pha-4(ts); htz-1(RNAi) worm at 20 °C missing a detectable pharynx. (F) Quantitation of pha-4(ts) animals exhibiting a normal pharynx (WT), an unattached or incomplete pharynx (Pun), or no detectable pharynx. htz-1, ssl-1, or mys-1 RNAi significantly increased the number of worms with no detectable pharynx (htz-1 and ssl-1: p < 0.0001; mys-1: p = 0.0015; Fisher exact test). WT worms with reduced htz-1, ssl-1, or mys-1 activity had a wild-type pharynx at 24 °C and 20 °C (unpublished data). RNAi was conducted by feeding dsRNA [29].
Figure 3. Specificity of mys-1, ssl-1, and htz-1 Synergy with pha-4(ts)
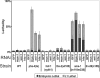
The indicated worm strains were fed dsRNA for GFP (negative control) or mys-1, ssl-1, or htz-1 at 20 °C (or 24 °C for WT and pha-4(ts)). Lethal embryos (black bars) or lethal L1 progeny (grey bars) were scored for each strain. Effectiveness of RNAi feeding was manifest through viable, but sterile, progeny for mys-1 and ssl-1 [14], as well as repeated enhancement of L1 lethality for pha-4(ts). n =1 00 worms/plate, three plates per column. Error bars indicate the standard deviation.
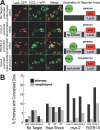
Figure 4. Association of YFP::HTZ-1 with Pharyngeal Promoters
(A) Extrachromosomal target arrays were visualized by LacI::CFP (red) bound to the Lac operator (LacO). YFP::HTZ-1 (green) was excluded (arrowheads) from arrays with no-target promoter (row 1), but associated with target arrays containing promoters for pharyngeal genes myo-2 or R07B1.9 in pharyngeal cells _(_rows 2 and 3). Merge is yellow. YFP::HTZ-1 was excluded from arrays containing the myo-2 promoter in non-pharyngeal cells (row 4). Cartoons depict interpretation of data. (B) Percentage of embryos containing one or more co-localized LacI::CFP and YFP::HTZ-1 dots in the pharynx (black) or outside of the pharynx (grey). Association was significantly higher in the three myo-2 promoter lines (p < 0.0001) and two R07B1.9 lines (_p_ = 0.0014). No significant difference in association was found when comparing the no-target lines to the heat-shock promoter target at 15 °C, 24 °C, or heat-shock at 33 °C for 30 min (_p_ > 0.095). More than 60 embryos were scored for each no-target, heat-shock, and R07B1.9 line. More than 120 embryos were scored for each myo-2 line. YFP::HTZ-1 association was predominantly pharyngeal for myo-2 (p < 0.0001) and R07B1.9 (p = 0.0407) target arrays, but not control arrays (p = 1.00). An equivalent number of images were taken at the 1.5-, 2-, and 3-fold stages of embryogenesis for each line. The _p_-values were calculated using Fisher exact test.
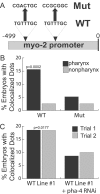
Figure 5. Association of YFP::HTZ-1 Depends on pha-4 Activity
(A) Two PHA-4 binding sites within the myo-2 promoter were mutated in the myo-2 Mut construct [18]. (B) YFP::HTZ-1 association in the pharynx (black) and outside of the pharynx (grey). n = 384 embryos for three wild-type (WT) lines and 192 embryos for three mutant (Mut) lines. _p_-value indicates a significant difference for pharyngeal association of YFP::HTZ-1 in WT versus Mut. (C) Association of YFP::HTZ-1 with the wild-type myo-2 promoter (line #1) decreased in pha-4(RNAi) embryos within pharyngeal cells. An equivalent number of images were taken at the 1.5-, 2-, and 3-fold stages of embryogenesis and >80 embryos were imaged for each trial. _p_-values calculated using Fisher's exact test.
Figure 6. YFP::HTZ-1 Association Peaks at the Onset of myo-2 Expression
(A) Association of YFP::HTZ-1 with the myo-2 promoter in the pharynx during the comma, 1.5-, 2-, and 3-fold stages of development (two trials: black, dark grey). YFP::HTZ-1 association in the pharynx does not peak at the 2-fold stage for a myo-2 promoter with both PHA-4 binding sites mutated (Mut, light grey). n > 60 embryos imaged at each stage, for each experiment. Multiple lines were used for each trial. (B) Pharyngeal YFP::HTZ-1 association with myo-2 target array #1 decreased at the 2-fold stage when pha-4 activity was reduced by RNAi_. n_ > 20 embryos imaged at each stage for each of the two wild-type (WT) and two pha-4(RNAi) experiments. The _p_-values for (A) and (B) indicate the significance of the WT peak at the 2-fold stage as calculated by a repeated measures analysis of variance (ANOVA).
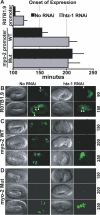
Figure 7. htz-1 Influences the Onset of Late Pharyngeal Gene Expression
(A) Onset of R07B1.9::GFP and _myo-2::_GFP after the comma stage for wild-type (black) or htz-1(RNAi) (grey) embryos. Activation of the R07B1.9 reporter was delayed after injection of htz-1 dsRNA (p < 0.0001, Student _t_-test). No RNAi: n = 18, htz-1 RNAi: n = 12 embryos. myo-2 activation was scored in embryos carrying either a wild-type myo-2::GFP reporter (WT) or a _myo-2::_GFP reporter with both PHA-4 binding sites mutated (Mut) [18]. Activation of the WT reporter was delayed after injection of htz-1 dsRNA (p < 0.0001, Student _t_-test), whereas onset of the Mut reporter was unchanged (p = 0.2068). No RNAi: n = 15 WT, n = 33 Mut Embryos; htz-1 RNAi: n = 9 WT, n = 21 Mut embryos. Error bars indicate the standard deviation. (B) Expression of R07B1.9::GFP at 90 and 180 min after the comma stage with htz-1 RNAi or without (No RNAi). Pharyngeal R07B1.9::GFP is indicated by arrowheads. (C) Expression of myo-2::GFP (WT) at 150, 200, and 250 min after the comma stage with htz-1 RNAi or without (No RNAi). (D) Expression of myo-2::GFP lacking PHA-4 binding sites [18] at 150, 200, 250 min after the comma stage with htz-1 RNAi or without (No RNAi).
Figure 8. htz-1 Influences the Onset of Early PHA-4–Dependent Expression
(A) Onset of 3XPRE::GFP after the two-cell stage for wild-type (black) or htz-1(RNAi) (grey) embryos_. 3XPRE::GFP_ is a reporter construct with three copies of a high-affinity PHA-4 response element upstream of the Δpes-10 promoter that reproducibly activates early pharyngeal expression [19]. Activation of the 3XPRE::GFP reporter is delayed after injection of htz-1 dsRNA (p = 0.0289, Student _t_-test). No RNAi: n = 23, htz-1 RNAi: n = 9 embryos. Error bars indicate the standard deviation. (B) Expression of 3XPRE::GFP at 150 and 200 min after the two-cell stage with htz-1 RNAi or without (No RNAi).
Figure 9. Summary and Model: HTZ-1 Synergizes with PHA-4 to Establish the Foregut
(A) Summary of the data presented in this paper and in [18]. myo-2 expression initiates at the 2-fold stage, and onset at this stage requires pha-4 and htz-1 because mutation of PHA-4 binding sites (Mut) or htz-1 RNAi lead to a delay in myo-2 activation. HTZ-1 association with the myo-2 promoter peaks at the onset of myo-2 transcription, and this association requires pha-4. (B) Model to explain how HTZ-1 synergizes with PHA-4. PHA-4 association with the myo-2 promoter leads to exchange of H2A-containing nucleosomes for one or more nucleosomes carrying HTZ-1/H2A.Z at the 2-fold stage. Based on data from other organisms [1], we propose MYS-1 and/or SSL-1 function in a complex that performs the exchange reaction. HTZ-1–containing nucleosomes promote transcriptional activation by the 2-fold stage.
Similar articles
- HTZ-1/H2A.z and MYS-1/MYST HAT act redundantly to maintain cell fates in somatic gonadal cells through repression of ceh-22 in C. elegans.
Shibata Y, Sawa H, Nishiwaki K. Shibata Y, et al. Development. 2014 Jan;141(1):209-18. doi: 10.1242/dev.090746. Development. 2014. PMID: 24346701 - Dynamic chromatin organization during foregut development mediated by the organ selector gene PHA-4/FoxA.
Fakhouri TH, Stevenson J, Chisholm AD, Mango SE. Fakhouri TH, et al. PLoS Genet. 2010 Aug 12;6(8):e1001060. doi: 10.1371/journal.pgen.1001060. PLoS Genet. 2010. PMID: 20714352 Free PMC article. - pha-4 is Ce-fkh-1, a fork head/HNF-3alpha,beta,gamma homolog that functions in organogenesis of the C. elegans pharynx.
Kalb JM, Lau KK, Goszczynski B, Fukushige T, Moons D, Okkema PG, McGhee JD. Kalb JM, et al. Development. 1998 Jun;125(12):2171-80. doi: 10.1242/dev.125.12.2171. Development. 1998. PMID: 9584117 - The molecular basis of organ formation: insights from the C. elegans foregut.
Mango SE. Mango SE. Annu Rev Cell Dev Biol. 2009;25:597-628. doi: 10.1146/annurev.cellbio.24.110707.175411. Annu Rev Cell Dev Biol. 2009. PMID: 19575642 Free PMC article. Review. - Temporal and spatial patterning of an organ by a single transcription factor.
Banerjee D, Slack FJ. Banerjee D, et al. Genome Biol. 2005;6(2):205. doi: 10.1186/gb-2005-6-2-205. Epub 2005 Jan 25. Genome Biol. 2005. PMID: 15693952 Free PMC article. Review.
Cited by
- Pioneer transcription factors in cell reprogramming.
Iwafuchi-Doi M, Zaret KS. Iwafuchi-Doi M, et al. Genes Dev. 2014 Dec 15;28(24):2679-92. doi: 10.1101/gad.253443.114. Genes Dev. 2014. PMID: 25512556 Free PMC article. Review. - An acetylated form of histone H2A.Z regulates chromosome architecture in Schizosaccharomyces pombe.
Kim HS, Vanoosthuyse V, Fillingham J, Roguev A, Watt S, Kislinger T, Treyer A, Carpenter LR, Bennett CS, Emili A, Greenblatt JF, Hardwick KG, Krogan NJ, Bähler J, Keogh MC. Kim HS, et al. Nat Struct Mol Biol. 2009 Dec;16(12):1286-93. doi: 10.1038/nsmb.1688. Epub 2009 Nov 15. Nat Struct Mol Biol. 2009. PMID: 19915592 Free PMC article. - H2A.Z marks antisense promoters and has positive effects on antisense transcript levels in budding yeast.
Gu M, Naiyachit Y, Wood TJ, Millar CB. Gu M, et al. BMC Genomics. 2015 Feb 19;16(1):99. doi: 10.1186/s12864-015-1247-4. BMC Genomics. 2015. PMID: 25765960 Free PMC article. - The Caenorhabditis elegans ekl (enhancer of ksr-1 lethality) genes include putative components of a germline small RNA pathway.
Rocheleau CE, Cullison K, Huang K, Bernstein Y, Spilker AC, Sundaram MV. Rocheleau CE, et al. Genetics. 2008 Mar;178(3):1431-43. doi: 10.1534/genetics.107.084608. Epub 2008 Feb 3. Genetics. 2008. PMID: 18245826 Free PMC article. - TRANSCRIPTION. Recruitment of RNA polymerase II by the pioneer transcription factor PHA-4.
Hsu HT, Chen HM, Yang Z, Wang J, Lee NK, Burger A, Zaret K, Liu T, Levine E, Mango SE. Hsu HT, et al. Science. 2015 Jun 19;348(6241):1372-6. doi: 10.1126/science.aab1223. Science. 2015. PMID: 26089518 Free PMC article.
References
- Kamakaka RT, Biggins S. Histone variants: Deviants? Genes Dev. 2005;19:295–310. - PubMed
- Stargell LA, Bowen J, Dadd CA, Dedon PC, Davis M, et al. Temporal and spatial association of histone H2A variant hv1 with transcriptionally competent chromatin during nuclear development in Tetrahymena thermophila . Genes Dev. 1993;7:2641–2651. - PubMed
- Allis CD, Richman R, Gorovsky MA, Ziegler YS, Touchstone B, et al. hv1 is an evolutionarily conserved H2A variant that is preferentially associated with active genes. J Biol Chem. 1986;261:1941–1948. - PubMed
- Meneghini MD, Wu M, Madhani HD. Conserved histone variant H2A.Z protects euchromatin from the ectopic spread of silent heterochromatin. Cell. 2003;112:725–736. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM056264/GM/NIGMS NIH HHS/United States
- 5T32 HD07491/HD/NICHD NIH HHS/United States
- T32 HD007491/HD/NICHD NIH HHS/United States
- 2P30CA42014/CA/NCI NIH HHS/United States
- P30 CA042014/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases