Transcriptional control of adipocyte formation - PubMed (original) (raw)
Review
Transcriptional control of adipocyte formation
Stephen R Farmer. Cell Metab. 2006 Oct.
Abstract
A detailed understanding of the processes governing adipose tissue formation will be instrumental in combating the obesity epidemic. Much progress has been made in the last two decades in defining transcriptional events controlling the differentiation of mesenchymal stem cells into adipocytes. A complex network of transcription factors and cell-cycle regulators, in concert with specific transcriptional coactivators and corepressors, respond to extracellular stimuli to activate or repress adipocyte differentiation. This review summarizes advances in this field, which constitute a framework for potential antiobesity strategies.
Figures
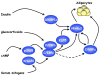
Figure 1. Induction of adipogenesis by a cascade of transcription factors
Exposure of preadipocytes to a cocktail of adipogenic inducers comprised of insulin, glucocorticoids, agents that elevate cAMP (isobutylmethylxanthine), and fetal bovine serum activates expression of several transcription factors that converge on PPARγ. PPARγ then induces C/EBPα expression, and together, these factors oversee terminal adipogenesis.
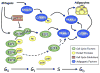
Figure 2. Role of cell-cycle proteins in regulating adipogenesis
An alternative pathway to that presented in Figure 1 exists whereby E2Fs and associated pocket proteins regulate expression of PPARγ1. Activation of PPARγ2 likely occurs through the induction of C/EBPα by PPARγ1, and C/EBPα then induces PPARγ2 expression. This model is consistent with a role for clonal expansion in promoting adipogenesis. G0, G1, and S correspond to phases of the cell cycle, while GD is a term used to define the growth-arrested state of terminally differentiated cells.
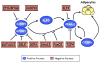
Figure 3. Negative control of adipogenesis
Negative regulators inhibit expression of PPARγ and C/EBPα by attenuating the activity of components of the cascade presented in Figure 1. Several of these negative factors appear to converge on C/EBPβ, supporting its role as a principal regulator of adipogenesis.
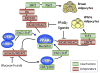
Figure 4. Coregulators and adipogenesis
The activity of the adipogenic transcription factors is regulated by association with various corepressors (red boxes) or coactivators (green boxes) at different stages of differentiation of both brown as well as white preadipocytes. The Mediator shown to be associating with PPARγ corresponds to the TRAP (thyroid hormone receptor-associated protein) transcriptional coactivator complex, which interacts physically with PPARγ through the TRAP220 subunit. See text for detailed discussion of the participation of the various protein complexes in controlling each of the transcription factors.
Similar articles
- Adipocyte differentiation from the inside out.
Rosen ED, MacDougald OA. Rosen ED, et al. Nat Rev Mol Cell Biol. 2006 Dec;7(12):885-96. doi: 10.1038/nrm2066. Nat Rev Mol Cell Biol. 2006. PMID: 17139329 Review. - Epigenetic modifications of the Zfp/ZNF423 gene control murine adipogenic commitment and are dysregulated in human hypertrophic obesity.
Longo M, Raciti GA, Zatterale F, Parrillo L, Desiderio A, Spinelli R, Hammarstedt A, Hedjazifar S, Hoffmann JM, Nigro C, Mirra P, Fiory F, Formisano P, Miele C, Smith U, Beguinot F. Longo M, et al. Diabetologia. 2018 Feb;61(2):369-380. doi: 10.1007/s00125-017-4471-4. Epub 2017 Oct 24. Diabetologia. 2018. PMID: 29067487 Free PMC article. - [Research advances on coregulators in adipocyte differentiation].
Wang N, Yan XH. Wang N, et al. Sheng Li Ke Xue Jin Zhan. 2009 Oct;40(4):308-12. Sheng Li Ke Xue Jin Zhan. 2009. PMID: 21417028 Review. Chinese. - The transcription factor NKX1-2 promotes adipogenesis and may contribute to a balance between adipocyte and osteoblast differentiation.
Chen N, Schill RL, O'Donnell M, Xu K, Bagchi DP, MacDougald OA, Koenig RJ, Xu B. Chen N, et al. J Biol Chem. 2019 Nov 29;294(48):18408-18420. doi: 10.1074/jbc.RA119.007967. Epub 2019 Oct 15. J Biol Chem. 2019. PMID: 31615896 Free PMC article. - Vestigial-like 3 is an inhibitor of adipocyte differentiation.
Halperin DS, Pan C, Lusis AJ, Tontonoz P. Halperin DS, et al. J Lipid Res. 2013 Feb;54(2):473-81. doi: 10.1194/jlr.M032755. Epub 2012 Nov 13. J Lipid Res. 2013. PMID: 23152581 Free PMC article.
Cited by
- Srebf1a is a key regulator of transcriptional control for adipogenesis.
Ayala-Sumuano JT, Velez-Delvalle C, Beltrán-Langarica A, Marsch-Moreno M, Cerbón-Solorzano J, Kuri-Harcuch W. Ayala-Sumuano JT, et al. Sci Rep. 2011;1:178. doi: 10.1038/srep00178. Epub 2011 Dec 1. Sci Rep. 2011. PMID: 22355693 Free PMC article. - The protective effects of steamed ginger on adipogenesis in 3T3-L1 cells and adiposity in diet-induced obese mice.
Kim B, Kim HJ, Cha YS. Kim B, et al. Nutr Res Pract. 2021 Jun;15(3):279-293. doi: 10.4162/nrp.2021.15.3.279. Epub 2021 Jan 12. Nutr Res Pract. 2021. PMID: 34093970 Free PMC article. - A20 reduces lipid storage and inflammation in hypertrophic adipocytes via p38 and Akt signaling.
Ai L, Wang X, Chen Z, Lin Q, Su D, Xu Q, Wu C, Jiang X, Xu A, Fan Z. Ai L, et al. Mol Cell Biochem. 2016 Sep;420(1-2):73-83. doi: 10.1007/s11010-016-2768-0. Epub 2016 Jul 22. Mol Cell Biochem. 2016. PMID: 27443844 - Bee (Apis mellifera L. 1758) wax restores adipogenesis and lipid accumulation of 3T3-L1 cells in cancer-associated cachexia condition.
Jang HJ, Kim HY, Lyu JH, Muthamil S, Shin UC, Kim HS, Jeong J, Chang S, Lee YK, Park JH. Jang HJ, et al. Food Sci Nutr. 2024 Apr 17;12(7):5027-5035. doi: 10.1002/fsn3.4153. eCollection 2024 Jul. Food Sci Nutr. 2024. PMID: 39055217 Free PMC article. - Histone demethylase Kdm4b functions as a co-factor of C/EBPβ to promote mitotic clonal expansion during differentiation of 3T3-L1 preadipocytes.
Guo L, Li X, Huang JX, Huang HY, Zhang YY, Qian SW, Zhu H, Zhang YD, Liu Y, Liu Y, Wang KK, Tang QQ. Guo L, et al. Cell Death Differ. 2012 Dec;19(12):1917-27. doi: 10.1038/cdd.2012.75. Epub 2012 Jun 22. Cell Death Differ. 2012. PMID: 22722334 Free PMC article.
References
- Arango NA, Szotek PP, Manganaro TF, Oliva E, Donahoe PK, Teixeira J. Conditional deletion of beta-catenin in the mesenchyme of the developing mouse uterus results in a switch to adipogenesis in the myometrium. Dev Biol. 2005;288:276–283. - PubMed
- Banerjee SS, Feinberg MW, Watanabe M, Gray S, Haspel RL, Denkinger DJ, Kawahara R, Hauner H, Jain MK. The Kruppel-like factor KLF2 inhibits peroxisome proliferator-activated receptor-gamma expression and adipogenesis. J Biol Chem. 2003;278:2581–2584. - PubMed
- Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR, Koder A, Evans RM. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell. 1999;4:585–595. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R56 DK051586/DK/NIDDK NIH HHS/United States
- R01 DK051586/DK/NIDDK NIH HHS/United States
- R01 DK058825/DK/NIDDK NIH HHS/United States
- DK58825/DK/NIDDK NIH HHS/United States
- R56 DK058825-06/DK/NIDDK NIH HHS/United States
- R01 DK051586-10/DK/NIDDK NIH HHS/United States
- DK51586/DK/NIDDK NIH HHS/United States
- R56 DK058825/DK/NIDDK NIH HHS/United States
- R01 DK058825-06A1/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources