Evidence for conformational changes within DsbD: possible role for membrane-embedded proline residues - PubMed (original) (raw)
Evidence for conformational changes within DsbD: possible role for membrane-embedded proline residues
Annie Hiniker et al. J Bacteriol. 2006 Oct.
Abstract
The mechanism by which DsbD transports electrons across the cytoplasmic membrane is unknown. Here we provide evidence that DsbD's conformation depends on its oxidation state. Our data also suggest that four highly conserved prolines surrounding DsbD's membrane-embedded catalytic cysteines may have an important functional role, possibly conferring conformational flexibility to DsbD.
Figures
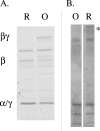
FIG. 1.
Protease sensitivities of reduced (lanes R) and oxidized (lanes O) DsbD. A. Digestion pattern of a DsbD construct presenting two thrombin sites inserted between α and β and between β and γ. After reduction of the protein with dithiothreitol, followed by gel filtration to remove the reductant, DsbD is cut into three polypeptides by thrombin (α and γ, which have the same molecular mass and are therefore visible as a single band of 15 kDa [marked α/γ], and β) (3). B. A significant amount of reduced DsbD (asterisk) is still uncut after a 10-min incubation at pH 7.8 with endoGlu-C, a protease which cleaves after glutamate residues under these conditions. There are 15 glutamate residues in DsbD.
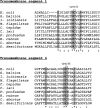
FIG. 2.
Multiple-sequence alignments of DsbDβ. The sequences of transmembrane segments 1 and 4 of E. coli DsbD (as predicted previously [5]) were aligned with the corresponding segments of DsbD homologues from Shewanella baltica, Idiomarina loihiensis, Methylobacillus flagellatus, Chlorobium tepidum, Campylobacter lari, Desulfovibrio desulfuricans, Chlamydophila abortus, Photobacterium profundum, and Rhodospirillum rubrum by using ClustalW. The conserved residues are shaded, an asterisk indicates that the residues in that column are identical in all sequences aligned, a colon indicates that conserved substitutions are present, and a period indicates that semiconserved substitutions are present.
FIG. 3.
A. Spot titers of wild-type (wt) and mutant DsbDs on copper plates. Strains were grown on plates containing copper (6 mM), ampicillin (200 μg/ml), and 40 μM IPTG to induce expression. A dsbA dsbD double mutant was used to express DsbD variants Pro289A, Pro284A, Pro166A, and Pro162A and wild-type DsbD from pTrc. B. Expression levels of the mutants and wild-type DsbD. BL21 cells expressing wild-type DsbD and variants were grown in LB, and protein expression was induced with IPTG. After a 4-h induction, cells were collected. Membrane pellets were prepared, and proteins were solubilized in 1% Triton. Expression levels were assessed by Western blot analysis using an anti-His tag antibody. Lanes: 1, wild type; 2, P162A, 3, P166A; 4, P284A; 5, P289A.
FIG. 4.
Redox states of wild-type DsbD* and variants. Cells expressing wild-type DsbD* and variants were grown in LB, and protein expression was induced with IPTG. After a 4-h induction, cells were collected and disrupted and membrane pellets were prepared. Proteins were solubilized in 1% Triton and free thiols were alkylated with MalPEG as described previously (10). Proteins were separated by SDS-polyacrylamide gel electrophoresis and visualized by Western blotting using anti-His tag antibodies. Oxidized protein (bands 1 and 2) and reduced protein (bands 3 and 4) are indicated. DTT, dithiothreitol.
Similar articles
- Thermodynamic aspects of DsbD-mediated electron transport.
Rozhkova A, Glockshuber R. Rozhkova A, et al. J Mol Biol. 2008 Jul 25;380(5):783-8. doi: 10.1016/j.jmb.2008.05.050. Epub 2008 May 29. J Mol Biol. 2008. PMID: 18571669 - Role and location of the unusual redox-active cysteines in the hydrophobic domain of the transmembrane electron transporter DsbD.
Katzen F, Beckwith J. Katzen F, et al. Proc Natl Acad Sci U S A. 2003 Sep 2;100(18):10471-6. doi: 10.1073/pnas.1334136100. Epub 2003 Aug 18. Proc Natl Acad Sci U S A. 2003. PMID: 12925743 Free PMC article. - Mutations of the membrane-bound disulfide reductase DsbD that block electron transfer steps from cytoplasm to periplasm in Escherichia coli.
Cho SH, Beckwith J. Cho SH, et al. J Bacteriol. 2006 Jul;188(14):5066-76. doi: 10.1128/JB.00368-06. J Bacteriol. 2006. PMID: 16816179 Free PMC article. - Many roles of the bacterial envelope reducing pathways.
Cho SH, Collet JF. Cho SH, et al. Antioxid Redox Signal. 2013 May 1;18(13):1690-8. doi: 10.1089/ars.2012.4962. Epub 2012 Nov 6. Antioxid Redox Signal. 2013. PMID: 23025488 Free PMC article. Review. - Basic and applied features of multicopper oxidases, CueO, bilirubin oxidase, and laccase.
Sakurai T, Kataoka K. Sakurai T, et al. Chem Rec. 2007;7(4):220-9. doi: 10.1002/tcr.20125. Chem Rec. 2007. PMID: 17663447 Review.
Cited by
- Disulfide Bond Formation in the Periplasm of Escherichia coli.
Manta B, Boyd D, Berkmen M. Manta B, et al. EcoSal Plus. 2019 Feb;8(2):10.1128/ecosalplus.ESP-0012-2018. doi: 10.1128/ecosalplus.ESP-0012-2018. EcoSal Plus. 2019. PMID: 30761987 Free PMC article. Review. - Structure-based modeling and engineering of Corynebacterium glutamicum LysE transporter for efficient extrusion of L-arginine.
Li C, Lv P, Feng L, Liu Y, Zhang Y, Peng Y, Li C, Yang C. Li C, et al. Commun Biol. 2025 Apr 2;8(1):543. doi: 10.1038/s42003-025-07997-x. Commun Biol. 2025. PMID: 40175674 Free PMC article. - The disulphide isomerase DsbC cooperates with the oxidase DsbA in a DsbD-independent manner.
Vertommen D, Depuydt M, Pan J, Leverrier P, Knoops L, Szikora JP, Messens J, Bardwell JC, Collet JF. Vertommen D, et al. Mol Microbiol. 2008 Jan;67(2):336-49. doi: 10.1111/j.1365-2958.2007.06030.x. Epub 2007 Nov 25. Mol Microbiol. 2008. PMID: 18036138 Free PMC article. - Protein Disulfide Exchange by the Intramembrane Enzymes DsbB, DsbD, and CcdA.
Bushweller JH. Bushweller JH. J Mol Biol. 2020 Aug 21;432(18):5091-5103. doi: 10.1016/j.jmb.2020.04.008. Epub 2020 Apr 16. J Mol Biol. 2020. PMID: 32305461 Free PMC article. Review. - Redox-active cysteines of a membrane electron transporter DsbD show dual compartment accessibility.
Cho SH, Porat A, Ye J, Beckwith J. Cho SH, et al. EMBO J. 2007 Aug 8;26(15):3509-20. doi: 10.1038/sj.emboj.7601799. Epub 2007 Jul 19. EMBO J. 2007. PMID: 17641688 Free PMC article.
References
- Collet, J. F., and J. C. Bardwell. 2002. Oxidative protein folding in bacteria. Mol. Microbiol. 44:1-8. - PubMed
- Collet, J. F., J. Riemer, M. W. Bader, and J. C. Bardwell. 2002. Reconstitution of a disulfide isomerization system. J. Biol. Chem. 277:26886-26892. - PubMed
- Cordes, F. S., J. N. Bright, and M. S. Sansom. 2002. Proline-induced distortions of transmembrane helices. J. Mol. Biol. 323:951-960. - PubMed
- Gordon, E. H., M. D. Page, A. C. Willis, and S. J. Ferguson. 2000. Escherichia coli DipZ: anatomy of a transmembrane protein disulphide reductase in which three pairs of cysteine residues, one in each of three domains, contribute differentially to function. Mol. Microbiol. 35:1360-1374. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases