Heterogeneity of ubiquitin pathology in frontotemporal lobar degeneration: classification and relation to clinical phenotype - PubMed (original) (raw)
Heterogeneity of ubiquitin pathology in frontotemporal lobar degeneration: classification and relation to clinical phenotype
Ian R A Mackenzie et al. Acta Neuropathol. 2006 Nov.
Abstract
We have investigated the extent and pattern of immunostaining for ubiquitin protein (UBQ) in 60 patients with frontotemporal lobar degeneration (FTLD) with ubiquitin-positive, tau-negative inclusions (FTLD-U), 37 of whom were ascertained in Manchester UK and 23 in Newcastle-Upon-Tyne, UK. There were three distinct histological patterns according to the form and distribution of the UBQ pathology. Histological type 1 was present in 19 patients (32%) and characterised by the presence of a moderate number, or numerous, UBQ immunoreactive neurites and intraneuronal cytoplasmic inclusions within layer II of the frontal and temporal cerebral cortex, and cytoplasmic inclusions within granule cells of the dentate gyrus; neuronal intranuclear inclusions (NII) of a "cat's eye" or "lentiform" appearance were present in 17 of these patients. In histological type 2 (16 patients, 27%), UBQ neurites were predominantly, or exclusively, present with few intraneuronal cytoplasmic inclusions within layer II of the cerebral cortex, while in histological type 3 (25 patients, 42%), UBQ intraneuronal cytoplasmic inclusions either within the cortical layer II or in the granule cells of the dentate gyrus, with few or no UBQ neurites, were seen. In neither of these latter two groups were NII present. The influence of histological type on clinical phenotype was highly significant with type 1 histology being associated clinically with cases of frontotemporal dementia (FTD) or progressive non-fluent aphasia (PNFA), type 2 histology with semantic dementia (SD), and type 3 histology with FTD, or FTD and motor neurone disease (MND).
Figures
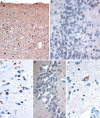
Fig. 1
Type 1 histology: In patient #1 there are numerous, UBQ immunoreactive neurites and intraneuronal cytoplasmic inclusions within layer II of the cerebral cortex (a) though UBQ cytoplasmic inclusions within granule cells of the dentate gyrus were relatively few (b). Neuronal intranuclear inclusions (NII) of a “cat’s eye” or “lentiform” appearance were also seen in pyramidal cells of layer II of cerebral cortex in this patient (c). Only in a single patient (patient #2) was a single NII seen within hippocampus dentate gyrus granule cells (d), and in another patient (patient #3) NII were seen within pyramidal cells in area CA4/5 of hippocampus (e). Immunoperoxidase-haematoxylin, ×20 (a), ×40 (b–e) microscope magnification
Fig. 2
Type 2 histology: In patients #22 and #19, there are numerous UBQ neurites predominantly, or exclusively, present within layer II of the cerebral cortex with few or no intraneuronal cytoplasmic inclusions (a and c, respectively). UBQ cytoplasmic inclusions within granule cells of the dentate gyrus were also numerous in patient #22 (b), but were less common in patient #19 (d) Immunoperoxidase-haematoxylin, ×20 (a, c), ×40 (b, d) microscope magnification
Fig. 3
Type 3 histology: In patient #28 with type 3a histology, UBQ neuronal cytoplasmic inclusions are predominantly, or exclusively, present within the brain with only relatively few UBQ neurites being present (a). UBQ inclusions were also sometimes numerous within granule cells of the dentate gyrus numerous (b). In patient #32 there were few neuronal UBQ cytoplasmic inclusions and neurites within the cerebral cortex (c), though UBQ cytoplasmic inclusions were widespread within granule cells of the hippocampus dentate gyrus (d). Immunoperoxidase-haematoxylin, ×20 (a, c), ×40 (b, d) microscope magnification
Comment in
- A harmonized classification system for FTLD-TDP pathology.
Mackenzie IR, Neumann M, Baborie A, Sampathu DM, Du Plessis D, Jaros E, Perry RH, Trojanowski JQ, Mann DM, Lee VM. Mackenzie IR, et al. Acta Neuropathol. 2011 Jul;122(1):111-3. doi: 10.1007/s00401-011-0845-8. Epub 2011 Jun 5. Acta Neuropathol. 2011. PMID: 21644037 Free PMC article. No abstract available.
Similar articles
- Histopathological changes underlying frontotemporal lobar degeneration with clinicopathological correlation.
Shi J, Shaw CL, Du Plessis D, Richardson AM, Bailey KL, Julien C, Stopford C, Thompson J, Varma A, Craufurd D, Tian J, Pickering-Brown S, Neary D, Snowden JS, Mann DM. Shi J, et al. Acta Neuropathol. 2005 Nov;110(5):501-12. doi: 10.1007/s00401-005-1079-4. Epub 2005 Oct 13. Acta Neuropathol. 2005. PMID: 16222525 - Ubiquitinated pathological lesions in frontotemporal lobar degeneration contain the TAR DNA-binding protein, TDP-43.
Davidson Y, Kelley T, Mackenzie IR, Pickering-Brown S, Du Plessis D, Neary D, Snowden JS, Mann DM. Davidson Y, et al. Acta Neuropathol. 2007 May;113(5):521-33. doi: 10.1007/s00401-006-0189-y. Epub 2007 Jan 12. Acta Neuropathol. 2007. PMID: 17219193 - TDP-43 in ubiquitinated inclusions in the inferior olives in frontotemporal lobar degeneration and in other neurodegenerative diseases: a degenerative process distinct from normal ageing.
Davidson Y, Amin H, Kelley T, Shi J, Tian J, Kumaran R, Lashley T, Lees AJ, DuPlessis D, Neary D, Snowden J, Akiyama H, Arai T, Hasegawa M, Bandopadhyay R, Sikkink S, Pickering-Brown S, Mann DM. Davidson Y, et al. Acta Neuropathol. 2009 Sep;118(3):359-69. doi: 10.1007/s00401-009-0526-z. Epub 2009 Mar 28. Acta Neuropathol. 2009. PMID: 19330339 - Frontotemporal lobar degeneration: clinical and pathological relationships.
Snowden J, Neary D, Mann D. Snowden J, et al. Acta Neuropathol. 2007 Jul;114(1):31-8. doi: 10.1007/s00401-007-0236-3. Epub 2007 Jun 14. Acta Neuropathol. 2007. PMID: 17569065 Review. - Frontotemporal lobar degeneration with ubiquitin-positive inclusions: a molecular genetic update.
van der Zee J, Gijselinck I, Pirici D, Kumar-Singh S, Cruts M, Van Broeckhoven C. van der Zee J, et al. Neurodegener Dis. 2007;4(2-3):227-35. doi: 10.1159/000101847. Neurodegener Dis. 2007. PMID: 17596717 Review.
Cited by
- Heterogeneity of cerebral TDP-43 pathology in sporadic amyotrophic lateral sclerosis: Evidence for clinico-pathologic subtypes.
Takeuchi R, Tada M, Shiga A, Toyoshima Y, Konno T, Sato T, Nozaki H, Kato T, Horie M, Shimizu H, Takebayashi H, Onodera O, Nishizawa M, Kakita A, Takahashi H. Takeuchi R, et al. Acta Neuropathol Commun. 2016 Jun 23;4(1):61. doi: 10.1186/s40478-016-0335-2. Acta Neuropathol Commun. 2016. PMID: 27338935 Free PMC article. - Imaging correlates of pathology in corticobasal syndrome.
Whitwell JL, Jack CR Jr, Boeve BF, Parisi JE, Ahlskog JE, Drubach DA, Senjem ML, Knopman DS, Petersen RC, Dickson DW, Josephs KA. Whitwell JL, et al. Neurology. 2010 Nov 23;75(21):1879-87. doi: 10.1212/WNL.0b013e3181feb2e8. Neurology. 2010. PMID: 21098403 Free PMC article. - TDP-43 variants of frontotemporal lobar degeneration.
Bigio EH. Bigio EH. J Mol Neurosci. 2011 Nov;45(3):390-401. doi: 10.1007/s12031-011-9545-z. Epub 2011 May 24. J Mol Neurosci. 2011. PMID: 21607722 Free PMC article. - Distinct white matter injury associated with medial temporal lobe atrophy in Alzheimer's versus semantic dementia.
Bejanin A, Desgranges B, La Joie R, Landeau B, Perrotin A, Mézenge F, Belliard S, de La Sayette V, Eustache F, Chételat G. Bejanin A, et al. Hum Brain Mapp. 2017 Apr;38(4):1791-1800. doi: 10.1002/hbm.23482. Epub 2016 Dec 16. Hum Brain Mapp. 2017. PMID: 27981671 Free PMC article. - Hippocampal sclerosis in advanced age: clinical and pathological features.
Nelson PT, Schmitt FA, Lin Y, Abner EL, Jicha GA, Patel E, Thomason PC, Neltner JH, Smith CD, Santacruz KS, Sonnen JA, Poon LW, Gearing M, Green RC, Woodard JL, Van Eldik LJ, Kryscio RJ. Nelson PT, et al. Brain. 2011 May;134(Pt 5):1506-18. doi: 10.1093/brain/awr053. Brain. 2011. PMID: 21596774 Free PMC article.
References
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1038/nature05016', 'is_inner': False, 'url': 'https://doi.org/10.1038/nature05016'}, {'type': 'PubMed', 'value': '16862116', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/16862116/'}\]}
- Baker M, Mackenzie IRA, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, Snowden J, Adamson J, Sadovnick AD, Rollinson S, Cannon A, Dwosh E, Neary D, Melquist S, Richardson A, Dickson D, Eriksen J, Robinson T, Zehr C, Dickey CA, Crook R, McGowan E, Mann D, Boeve B, Feldman H, Hutton M (2006) Mutations in Progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 442:916–919 - PubMed
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1007/s004010050505', 'is_inner': False, 'url': 'https://doi.org/10.1007/s004010050505'}, {'type': 'PubMed', 'value': '8841663', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/8841663/'}\]}
- Bergmann M, Kuchelmeister K, Schmid KW, Kretzschmar HA, Schroder R (1996) Different variants of frontotemporal dementia: a neuropathological and immunohistochemical study. Acta Neuropathol 92:170–179 - PubMed
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '15330335', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/15330335/'}\]}
- Bigio EH, Johnson NA, Rademaker AW, Fung BB, Mesulam M-M, Siddique N, Dellefave L, Caliendo J, Freeman S, Siddique T (2004) Neuronal ubiquitinated intranuclear inclusions in familial and non-familial frontotemporal dementia of the motor neurone disease type associated with amyotrophic lateral sclerosis. J Neuropathol Exp Neurol 63: 810–811 - PubMed
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '0', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/0/'}\]}
- Brun A, Englund E, Gustafson L, Passant U, Mann DMA, Neary D, Snowden JS (1994) Clinical, neuropsychological and neuropathological criteria for fronto-temporal dementia. J Neurol Neurosurg Psychiatry 57:416–418 - PubMed
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1038/nature05017', 'is_inner': False, 'url': 'https://doi.org/10.1038/nature05017'}, {'type': 'PubMed', 'value': '16862115', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/16862115/'}\]}
- Cruts M, Gijselinck I, van der Zee J, Engelborghs S, Wils H, Pirici D, Rademakers R, Vandenberghe R, Dermaut B, Martin J-J, van Duijn C, Peeters K, Sciot R, Santens P, De Pooter T, Mattheijssens M, Van den Broeck M, Cujit I, Vennekens K, De Deyn PP, Kumar-Singh S, Van Broeckhoven C (2006) Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature 442:920–924 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous