Age-related changes in human crystallins determined from comparative analysis of post-translational modifications in young and aged lens: does deamidation contribute to crystallin insolubility? - PubMed (original) (raw)
Comparative Study
. 2006 Oct;5(10):2554-66.
doi: 10.1021/pr050473a.
Affiliations
- PMID: 17022627
- PMCID: PMC2536618
- DOI: 10.1021/pr050473a
Comparative Study
Age-related changes in human crystallins determined from comparative analysis of post-translational modifications in young and aged lens: does deamidation contribute to crystallin insolubility?
P A Wilmarth et al. J Proteome Res. 2006 Oct.
Abstract
We have employed recently developed blind modification search techniques to generate the most comprehensive map of post-translational modifications (PTMs) in human lens constructed to date. Three aged lenses, two of which had moderate cataract, and one young control lens were analyzed using multidimensional liquid chromatography mass spectrometry. In total, 491 modification sites in lens proteins were identified. There were 155 in vivo PTM sites in crystallins: 77 previously reported sites and 78 newly detected PTM sites. Several of these sites had modifications previously undetected by mass spectrometry in lens including carboxymethyl lysine (+58 Da), carboxyethyl lysine (+72 Da), and an arginine modification of +55 Da with yet unknown chemical structure. These new modifications were observed in all three aged lenses but were not found in the young lens. Several new sites of cysteine methylation were identified indicating this modification is more extensive in lens than previously thought. The results were used to estimate the extent of modification at specific sites by spectral counting. We tested the long-standing hypothesis that PTMs contribute to age-related loss of crystallin solubility by comparing spectral counts between the water-soluble and water-insoluble fractions of the aged lenses and found that the extent of deamidation was significantly increased in the water-insoluble fractions. On the basis of spectral counting, the most abundant PTMs in aged lenses were deamidations and methylated cysteines with other PTMs present at lower levels.
Figures
Figure 1
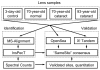
Workflow to identify post-translational modifications in lens proteins. Samples were analyzed with an unrestrictive (“blind”) search (MS-Alignment) in order to discover novel chemical modifications. A restrictive search (InsPecT) was then performed to increase sensitivity. Modifications were validated by looking for consensus across three search tools (InsPecT, OpenSea, and X! Tandem), and quantified using spectral counting.
Figure 2
Modification sites identified in (A) αA crystallin and (B) αB crystallin. Modified residues are bolded and the mass shifts of the modifications are specified above the residues.
Figure 3
Modification sites identified in (A) βA3/A1 crystallin, (B) βA4 crystallin, (C) βB1 crystallin, and (D) βB2 crystallin. Modified residues are bolded and the mass shifts of the modifications are specified above the residues.
Figure 4
Modification sites identified in (A) γS crystallin, (B) γC crystallin, and (C) γD crystallin. Modified residues are bolded and the mass shifts of the modifications are specified above the residues.
Figure 5
Tandem mass spectra used to assign the carboxymethyl lysine modification to K117 in βB1 peptide GEMFILEKGEYPR. The matched y-ions (in blue) and b-ions (in red) are highlighted and labeled. Panel A shows the unmodified, missed-cleavage peptide, which eluted in SCX fraction 42, for the 70-year old normal human water-insoluble lens sample. Panel B is the modified peptide (GEMFILEK+58GEYPR) from the same lens sample, which eluted in the earlier SCX fraction 33 indicating a modified basic site. Panel C is the same modified peptide from the 93-year old cataractous water-soluble lens sample observed with the QTOF instrument. The modification mass shift was 57.99 Da. In panels B and C, all y-ions (shown in blue) starting with y6 are clearly shifted by +58, and the modification can be unambiguously assigned to K117.
Figure 6
Ion trap tandem mass spectra of peptides containing the arginine +55 Da modifications are shown for (A) αA R12 in peptide R+55TLGPFYPSR, (B) γS R78 in peptide WMGLNDR+55LSSCR, and (C) βB1 R85 in peptide AEFSGECSNLADR+55GFDR. The spectra are from the 70-year old normal lens, the 70-year old cataractous lens, and the 93-year old cataractous lens, respectively. In all cases the modification caused a missed trypsin cleavage. Matched y- (in blue) and b-ions (in red) are highlighted and labeled.
Figure 7
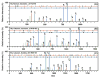
A comparison of the averaged relative deamidation abundances between water-soluble and water-insoluble proteins from the three aged lenses. Abundances were estimated from spectral counts and only the sites having a 2-fold or greater abundance difference are shown. The data shows not only increased deamidation present in the water-insoluble lens proteins, but also widely different extents of deamidation depending on the site.
Figure 8
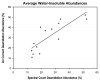
Correlation between average deamidation abundances determined from integrated ion currents and from spectral counting for the three aged lens water-insoluble fractions at 14 different crystallin deamidation sites.
Similar articles
- Modifications of the water-insoluble human lens alpha-crystallins.
Lund AL, Smith JB, Smith DL. Lund AL, et al. Exp Eye Res. 1996 Dec;63(6):661-72. doi: 10.1006/exer.1996.0160. Exp Eye Res. 1996. PMID: 9068373 - Resistance of human betaB2-crystallin to in vivo modification.
Zhang Z, David LL, Smith DL, Smith JB. Zhang Z, et al. Exp Eye Res. 2001 Aug;73(2):203-11. doi: 10.1006/exer.2001.1023. Exp Eye Res. 2001. PMID: 11446770 - One-shot LC-MS/MS analysis of post-translational modifications including oxidation and deamidation of rat lens α- and β-crystallins induced by γ-irradiation.
Kim I, Saito T, Fujii N, Kanamoto T, Fujii N. Kim I, et al. Amino Acids. 2016 Dec;48(12):2855-2866. doi: 10.1007/s00726-016-2324-y. Epub 2016 Sep 6. Amino Acids. 2016. PMID: 27600614 - The Proteome of Cataract Markers: Focus on Crystallins.
Zhang K, Zhu X, Lu Y. Zhang K, et al. Adv Clin Chem. 2018;86:179-210. doi: 10.1016/bs.acc.2018.05.005. Epub 2018 Jul 13. Adv Clin Chem. 2018. PMID: 30144840 Review. - Lens β-crystallins: the role of deamidation and related modifications in aging and cataract.
Lampi KJ, Wilmarth PA, Murray MR, David LL. Lampi KJ, et al. Prog Biophys Mol Biol. 2014 Jul;115(1):21-31. doi: 10.1016/j.pbiomolbio.2014.02.004. Epub 2014 Mar 6. Prog Biophys Mol Biol. 2014. PMID: 24613629 Free PMC article. Review.
Cited by
- Molecular Processes Implicated in Human Age-Related Nuclear Cataract.
Truscott RJW, Friedrich MG. Truscott RJW, et al. Invest Ophthalmol Vis Sci. 2019 Dec 2;60(15):5007-5021. doi: 10.1167/iovs.19-27535. Invest Ophthalmol Vis Sci. 2019. PMID: 31791064 Free PMC article. Review. - Large-scale binding of α-crystallin to cell membranes of aged normal human lenses: a phenomenon that can be induced by mild thermal stress.
Friedrich MG, Truscott RJ. Friedrich MG, et al. Invest Ophthalmol Vis Sci. 2010 Oct;51(10):5145-52. doi: 10.1167/iovs.10-5261. Epub 2010 Apr 30. Invest Ophthalmol Vis Sci. 2010. PMID: 20435594 Free PMC article. - Promotion of Protein Solubility and Reduction in Stiffness in Human Lenses by Aggrelyte-1: Implications for Reversing Presbyopia.
Panja S, Gaikwad H, Rankenberg J, Nam MH, Nagaraj RH. Panja S, et al. Int J Mol Sci. 2023 Jan 22;24(3):2196. doi: 10.3390/ijms24032196. Int J Mol Sci. 2023. PMID: 36768517 Free PMC article. - Eye lens β-crystallins are predicted by native ion mobility-mass spectrometry and computations to form compact higher-ordered heterooligomers.
Rolland AD, Takata T, Donor MT, Lampi KJ, Prell JS. Rolland AD, et al. Structure. 2023 Sep 7;31(9):1052-1064.e3. doi: 10.1016/j.str.2023.06.013. Epub 2023 Jul 14. Structure. 2023. PMID: 37453416 Free PMC article. - Human γS-Crystallin Resists Unfolding Despite Extensive Chemical Modification from Exposure to Ionizing Radiation.
Norton-Baker B, Rocha MA, Granger-Jones J, Fishman DA, Martin RW. Norton-Baker B, et al. J Phys Chem B. 2022 Jan 27;126(3):679-690. doi: 10.1021/acs.jpcb.1c08157. Epub 2022 Jan 13. J Phys Chem B. 2022. PMID: 35021623 Free PMC article.
References
- Lampi KJ, Ma Z, Shih M, Shearer TR, Smith JB, Smith DL, David LL. Sequence analysis of betaA3, betaB3, and betaA4 crystallins completes the identification of the major proteins in young human lens. J Biol Chem. 1997;272(4):2268–75. - PubMed
- Searle BC, Dasari S, Turner M, Reddy AP, Choi D, Wilmarth PA, McCormack AL, David LL, Nagalla SR. High-throughput identification of proteins and unanticipated sequence modifications using a mass-based alignment algorithm for MS/MS de novo sequencing results. Anal Chem. 2004;76(8):2220–30. - PubMed
- Searle BC, Dasari S, Wilmarth PA, Turner M, Reddy AP, David LL, Nagalla SR. Identification of protein modifications using MS/MS de novo sequencing and the OpenSea alignment algorithm. J Proteome Res. 2005;4(2):546–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 EY010572-149004/EY/NEI NIH HHS/United States
- R01 EY007755/EY/NEI NIH HHS/United States
- P30 EY010572/EY/NEI NIH HHS/United States
- R01 RR016522/RR/NCRR NIH HHS/United States
- EY007755/EY/NEI NIH HHS/United States
- U19 ES011384/ES/NIEHS NIH HHS/United States
- U19ES11384/ES/NIEHS NIH HHS/United States
- R01 EY007755-16/EY/NEI NIH HHS/United States
- R01 RR016522-05/RR/NCRR NIH HHS/United States
- EY10572/EY/NEI NIH HHS/United States
- 1-R01-RR16522/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous