L-arginine availability regulates T-lymphocyte cell-cycle progression - PubMed (original) (raw)
L-arginine availability regulates T-lymphocyte cell-cycle progression
Paulo C Rodriguez et al. Blood. 2007.
Abstract
L-arginine (L-Arg) plays a central role in several biologic systems including the regulation of T-cell function. L-Arg depletion by myeloid-derived suppressor cells producing arginase I is seen in patients with cancer inducing T-cell anergy. We studied how L-Arg starvation could regulate T-cell-cycle progression. Stimulated T cells cultured in the absence of L-Arg are arrested in the G0-G1phase of the cell cycle. This was associated with an inability of T cells to up-regulate cyclin D3 and cyclin-dependent kinase 4 (cdk4), but not cdk6, resulting in an impaired downstream signaling with a decreased phosphorylation of Rb protein and a low expression and binding of E2F1. Silencing of cyclin D3 reproduced the cell cycle arrest caused by L-Arg starvation. The regulation of cyclin D3 and cdk4 by L-Arg starvation occurs at transcriptional and posttranscriptional levels. Signaling through GCN2 kinase is triggered during amino acid starvation. Experiments demonstrated that T cells from GCN2 knock-out mice did not show a decreased proliferation and were able to up-regulate cyclin D3 when cultured in the absence of L-Arg. These results contribute to the understanding of a central mechanism by which cancer and other diseases characterized by high arginase I production may cause T-cell dysfunction.
Figures
Figure 1
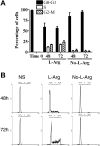
Decreased proliferation in stimulated T cells cultured in the absence of l-Arg. (A) Freshly isolated human T cells (5 × 105) stimulated with anti-CD3 plus anti-CD28 were cultured in the presence or the absence of
l
-Arg, and uptake of [3H]-thymidine was measured at 24, 48, 72, and 96 hours. (B) Stimulated T cells (5 × 105) were labeled with 1 μM CFSE and cultured in the presence or the absence of
l
-Arg; fluorescence was measured at 72 and 96 hours. Unstimulated (NS) control cells were CFSE-labeled T cells cultured in medium containing
l
-Arg. (C) Stimulated T cells (5 × 105) cultured in the absence of
l
-Arg for 24 hours were replenished with 2 mM
l
-lysine,
l
-glutamine,
l
-citrulline, or
l
-Arg, and incorporation of [3H]-thymidine was measured at 72 hours.
Figure 2
l-Arg starvation arrests T cells in G0-G1 phase of cell cycle. (A) Human T cells (5 × 105) were stimulated with anti-CD3 plus anti-CD28 in the presence or the absence of
l
-Arg, and cell cycle was assessed at 48 and 72 hours by flow cytometry using propidium iodide. Percentages of T cells at each cell-cycle phase from 5 different experiments were determined. (B) Representative experiments from panel A. Unstimulated control T cells (NS, left row) were in medium containing
l
-Arg.
Figure 3
Impaired expression of cyclin D3 and cdk4 in stimulated T cells cultured in the absence of l-Arg. (A-B) Whole-cell lysates were obtained from 3 × 106 activated T cells cultured in the presence or the absence of
l
-Arg for various lengths of time (hours) or from activated T cells cultured in the absence of
l
-Arg for 24 hours and then replenished with
l
-Arg at 48 or 72 hours. The expression of cyclin and cdks was tested by Western blotting. (C) Lysates from stimulated T cells cultured for 24 hours were prepared and 100 μg was immunoprecipitated with agarose-conjugated anti–cyclin D3 and anti-cdk4. Immunoprecipitates were then tested for cyclin D3 and cdk4 expression by Western blotting.
Figure 4
Activated T cells cultured in the absence of l-Arg have a decreased Rb phosphorylation and a decreased ability to phosphorylate Rb in vitro. (A) Whole-cell lysates obtained from stimulated T cells cultured for 24 hours in the presence or the absence of
l
-Arg were immunoblotted against Rb and vinculin. (B) Lysates from unstimulated or stimulated T cells cultured for 24 hours in the presence or the absence of
l
-Arg were prepared, and 100 μg was immunoprecipitated with agarose-conjugated anti–cyclin D3 and incubated with a reaction cocktail containing 0.2 μg GST-Rb and 10 μCi (0.37 MBq) [γ-32P] ATP. (C) Similarly, 100 μg whole-cell lysates from stimulated T cells cultured for 24 hours in medium containing
l
-Arg was initially immunodepleted with an irrelevant protein G (NRS), or agarose-conjugated antibodies against cyclin D3, cdk4, or cdk6, and then immunoprecipitated with irrelevant protein G (NRS) or anti–cyclin D3 and tested for kinase activity.
Figure 5
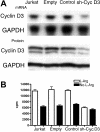
Cyclin D3 silencing mimics l-Arg starvation effects on cell cycle. (A) Wild-type Jurkat cells (Jurkat) and Jurkat cells transfected with empty vector, nonrelated human sequence, or sh–cyclin D3 plasmid were tested for the expression of cyclin D3 RNA by Northern blot and cyclin D3 protein by Western blot. (B) Wild-type Jurkat cells or transfected cells were cultured in the presence or the absence of
l
-Arg and tested for proliferation after 48 hours by [3H]-thymidine incorporation.
Figure 6
l
-Arg starvation impairs expression of cyclin D3 and cdk4 through transcriptional, posttranscriptional, and translational mechanisms. (A) RNA (5 μg) from nonactivated human T cells or T cells activated with anti-CD3 plus anti-CD28 and cultured in the presence or the absence of
l
-Arg was tested for cyclin D3, cdk4, and cdk6 RNA expression by Northern blot. (B) Nuclei obtained from activated T cells cultured in the presence and the absence of
l
-Arg were tested for cyclin D3, cdk4, and cdk6 transcriptional rate by run-on analysis as described in “Materials and methods.” (C) RNA stability was also tested in activated T cells cultured in the presence and the absence of
l
-Arg for 12 hours, after which Act D (5 μg/mL) was added and RNA collected after 3, 6, 12, 24, and 36 hours. RNA expression was then tested by Northern blot. (D) Nonactivated (NS, lane 1) and activated (lanes 2-3) T cells were cultured in the presence or the absence of
l
-Arg for 48 hours, after which cells were washed and pulsed with 250 μCi (9.25 MBq) [35S] methionine for 3 hours. Lysates were immunoprecipitated using anti–cyclin D3 as described in “Materials and methods.”
Figure 7
T cells from GCN2 KO mice proliferate in the absence of l-Arg. (A) T cells (2 × 105) were activated with bound anti-CD3 plus anti-CD28, and thymidine incorporation was tested after 48 hours (cpm ± SD). (B) Kinetics of expression of cyclin D3 and cdk4 in activated T cells (4 × 106) from GCN2 knock-out mice and congeneic wild-type mice.
Similar articles
- L-arginine deprivation regulates cyclin D3 mRNA stability in human T cells by controlling HuR expression.
Rodriguez PC, Hernandez CP, Morrow K, Sierra R, Zabaleta J, Wyczechowska DD, Ochoa AC. Rodriguez PC, et al. J Immunol. 2010 Nov 1;185(9):5198-204. doi: 10.4049/jimmunol.1001224. Epub 2010 Oct 1. J Immunol. 2010. PMID: 20889542 Free PMC article. - Failure of T lymphocytes from elderly humans to enter the cell cycle is associated with low Cdk6 activity and impaired phosphorylation of Rb protein.
Arbogast A, Boutet S, Phelouzat MA, Plastre O, Quadri R, Proust JJ. Arbogast A, et al. Cell Immunol. 1999 Oct 10;197(1):46-54. doi: 10.1006/cimm.1999.1550. Cell Immunol. 1999. PMID: 10555995 - Induction of G1 arrest by down-regulation of cyclin D3 in T cell hybridomas.
Miyatake S, Nakano H, Park SY, Yamazaki T, Takase K, Matsushime H, Kato A, Saito T. Miyatake S, et al. J Exp Med. 1995 Aug 1;182(2):401-8. doi: 10.1084/jem.182.2.401. J Exp Med. 1995. PMID: 7629502 Free PMC article. - [Molecular mechanisms controlling the cell cycle: fundamental aspects and implications for oncology].
Viallard JF, Lacombe F, Belloc F, Pellegrin JL, Reiffers J. Viallard JF, et al. Cancer Radiother. 2001 Apr;5(2):109-29. doi: 10.1016/s1278-3218(01)00087-7. Cancer Radiother. 2001. PMID: 11355576 Review. French. - Metabolism of L-arginine by myeloid-derived suppressor cells in cancer: mechanisms of T cell suppression and therapeutic perspectives.
Raber P, Ochoa AC, Rodríguez PC. Raber P, et al. Immunol Invest. 2012;41(6-7):614-34. doi: 10.3109/08820139.2012.680634. Immunol Invest. 2012. PMID: 23017138 Free PMC article. Review.
Cited by
- Cytotoxicity of tumor antigen specific human T cells is unimpaired by arginine depletion.
Munder M, Engelhardt M, Knies D, Medenhoff S, Wabnitz G, Luckner-Minden C, Feldmeyer N, Voss RH, Kropf P, Müller I, Conradi R, Samstag Y, Theobald M, Ho AD, Goldschmidt H, Hundemer M. Munder M, et al. PLoS One. 2013 May 23;8(5):e63521. doi: 10.1371/journal.pone.0063521. Print 2013. PLoS One. 2013. PMID: 23717444 Free PMC article. - Immune suppression in gliomas.
Grabowski MM, Sankey EW, Ryan KJ, Chongsathidkiet P, Lorrey SJ, Wilkinson DS, Fecci PE. Grabowski MM, et al. J Neurooncol. 2021 Jan;151(1):3-12. doi: 10.1007/s11060-020-03483-y. Epub 2020 Jun 15. J Neurooncol. 2021. PMID: 32542437 Free PMC article. Review. - Glioma-Immune Cell Crosstalk in Tumor Progression.
Elguindy M, Young JS, Mondal I, Lu RO, Ho WS. Elguindy M, et al. Cancers (Basel). 2024 Jan 11;16(2):308. doi: 10.3390/cancers16020308. Cancers (Basel). 2024. PMID: 38254796 Free PMC article. Review. - Pharmacological modulation of myeloid-derived suppressor cells to dampen inflammation.
van Geffen C, Heiss C, Deißler A, Kolahian S. van Geffen C, et al. Front Immunol. 2022 Aug 30;13:933847. doi: 10.3389/fimmu.2022.933847. eCollection 2022. Front Immunol. 2022. PMID: 36110844 Free PMC article. Review. - Altered Metabolism in Glioblastoma: Myeloid-Derived Suppressor Cell (MDSC) Fitness and Tumor-Infiltrating Lymphocyte (TIL) Dysfunction.
Di Ianni N, Musio S, Pellegatta S. Di Ianni N, et al. Int J Mol Sci. 2021 Apr 24;22(9):4460. doi: 10.3390/ijms22094460. Int J Mol Sci. 2021. PMID: 33923299 Free PMC article. Review.
References
- Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5:641–654. - PubMed
- Zea AH, Rodriguez PC, Atkins MB, et al. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res. 2005;65:3044–3048. - PubMed
- Roth E, Steininger R, Winkler S, et al. L-Arginine deficiency after liver transplantation as an effect of arginase efflux from the graft: influence on nitric oxide metabolism. Transplantation. 1994;57:665–669. - PubMed
- Angele MK, Smail N, Ayala A, et al. L-arginine: a unique amino acid for restoring the depressed macrophage functions after trauma-hemorrhage. J Trauma. 1999;46:34–41. - PubMed
- Barbul A. Arginine and immune function. Nutrition. 1990;6:53–58. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources