Secretory PLA2-IIA: a new inflammatory factor for Alzheimer's disease - PubMed (original) (raw)
Secretory PLA2-IIA: a new inflammatory factor for Alzheimer's disease
Guna S D Moses et al. J Neuroinflammation. 2006.
Abstract
Secretory phospholipase A2-IIA (sPLA2-IIA) is an inflammatory protein known to play a role in the pathogenesis of many inflammatory diseases. Although this enzyme has also been implicated in the pathogenesis of neurodegenerative diseases, there has not been a direct demonstration of its expression in diseased human brain. In this study, we show that sPLA2-IIA mRNA is up-regulated in Alzheimer's disease (AD) brains as compared to non-demented elderly brains (ND). We also report a higher percentage of sPLA2-IIA-immunoreactive astrocytes present in AD hippocampus and inferior temporal gyrus (ITG). In ITG, the majority of sPLA2-IIA-positive astrocytes were associated with amyloid beta (Abeta)-containing plaques. Studies with human astrocytes in culture demonstrated the ability of oligomeric Abeta1-42 and interleukin-1beta (IL-1beta) to induce sPLA2-IIA mRNA expression, indicating that this gene is among those induced by inflammatory cytokines. Since exogenous sPLA2-IIA has been shown to cause neuronal injury, understanding the mechanism(s) and physiological consequences of sPLA2-IIA upregulation in AD brain may facilitate the development of novel therapeutic strategies to inhibit the inflammatory responses and to retard the progression of the disease.
Figures
Figure 1
sPLA2-IIA immunoreactivity in human postmortem brain tissues. Double immunostaining depicting sPLA2-IIA immunoreactivity in dark blue color and GFAP immunoreactivity in brown color is shown in panels A-D (using 20X and 40X objective lenses). Panel A demonstrates that little sPLA2-IIA immunoreactivity is present in a cluster of GFAP immunoreactive astrocytes in ND hippocampus. Panel B shows many GFAP-positive astrocytes (white arrow) labeled with intense immunoreactivity for sPLA2-IIA (dark immunoreactive products, red arrow) in AD hippocampus. At higher magnification (Panel C), sPLA2-IIA immunoreactivity is shown in an astrocyte cell body in granular-like structures (red arrow). Panel D shows that immunoreactivity for sPLA2-IIA (red arrows) is also present in GFAP-positive astrcoytes (white arrows) surrounding microvessels in AD hippocampus. We also detected sPLA2 immunoreactivity in hippocampal neurons (black arrows) in ND (Panel A) and AD (Panel D) hippocampus. In Panel E, several sPLA2-IIA immunoreactivitve profiles (red arrows) are co-localized with an amyloid plaque (brown immunoreactive area) detected by immunohistochemistry with an antibody to Aβ.
Figure 2
Co-localization of sPLA2-IIA-positive astrocytes with thioflavin S-positive plaques. Double immunostaining of sPLA2-IIA and GFAP combined with thioflavin S staining shows the presence of sPLA2-IIA (red arrows) in GFAP-positive astrocytes (panels A and B) and their association with thioflavin S-positive amyloid plaques (green fluorescent area in panel A) in an ITG section from an AD case.
Figure 3
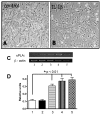
Induction of sPLA2-IIA mRNA expression by cytokines and Aβ 1–42 in cultured human astrocytes. Phase contrast micrographs show human astrocytes in control (panel A) and IL-1β-stimulated cultures (panel B) for 24 hours. Human postmortem astrocytes were used for the sPLA2-IIA RNA study. Experiments were performed using cultures derived from 3 neuropathologically confirmed AD cases. A representative gel depicting PCR-amplified fragments for sPLA2-IIA and β-actin is shown in panel C. Gel lanes 1–5 represent the following treatments used in the astrocyte cultures: 1. control; 2. IFNγ (100 ng/ml); 3. Aβ1–42 (2.5 μM); 4. IL-1β (20 ng/ml); 5. IL-1β and Aβ1–42. Twenty-four hours after treatment, RNA was extracted from cells, reverse transcribed, and RT-PCR was carried out as described in methods. Panel D shows a bar graph depicting relative units of sPLA2-IIA expression after normalization with β-actin. Significant differences (*) comparing treatment groups with controls were obtained by one-way ANOVA followed by Tukey multiple comparison post hoc test.
Similar articles
- Oleanolic acid protects against cognitive decline and neuroinflammation-mediated neurotoxicity by blocking secretory phospholipase A2 IIA-activated calcium signals.
Zhang L, Xia R, Jia J, Wang L, Li K, Li Y, Zhang J. Zhang L, et al. Mol Immunol. 2018 Jul;99:95-103. doi: 10.1016/j.molimm.2018.04.015. Epub 2018 May 7. Mol Immunol. 2018. PMID: 29747052 - Astrocytes regulate α-secretase-cleaved soluble amyloid precursor protein secretion in neuronal cells: Involvement of group IIA secretory phospholipase A2.
Yang X, Sheng W, Ridgley DM, Haidekker MA, Sun GY, Lee JC. Yang X, et al. Neuroscience. 2015 Aug 6;300:508-17. doi: 10.1016/j.neuroscience.2015.05.052. Epub 2015 May 30. Neuroscience. 2015. PMID: 26037803 Free PMC article. - Involvement of oxidative pathways in cytokine-induced secretory phospholipase A2-IIA in astrocytes.
Jensen MD, Sheng W, Simonyi A, Johnson GS, Sun AY, Sun GY. Jensen MD, et al. Neurochem Int. 2009 Nov;55(6):362-8. doi: 10.1016/j.neuint.2009.04.002. Epub 2009 Apr 16. Neurochem Int. 2009. PMID: 19375465 Free PMC article. - Effects of Statins and Xuezhikang on the Expression of Secretory Phospholipase A2, Group IIA in Rat Vascular Smooth Muscle Cells.
Xie Q, Zhang D. Xie Q, et al. Int Heart J. 2017 Feb 7;58(1):115-124. doi: 10.1536/ihj.16-163. Epub 2017 Jan 24. Int Heart J. 2017. PMID: 28123160 - The role of secretory phospholipase A₂ in the central nervous system and neurological diseases.
Yagami T, Yamamoto Y, Koma H. Yagami T, et al. Mol Neurobiol. 2014 Apr;49(2):863-76. doi: 10.1007/s12035-013-8565-9. Epub 2013 Oct 10. Mol Neurobiol. 2014. PMID: 24113843 Review.
Cited by
- Low molecular weight phospholipases A2 in mammalian brain and neural cells: roles in functions and dysfunctions.
Goracci G, Ferrini M, Nardicchi V. Goracci G, et al. Mol Neurobiol. 2010 Jun;41(2-3):274-89. doi: 10.1007/s12035-010-8108-6. Epub 2010 Mar 19. Mol Neurobiol. 2010. PMID: 20238205 Review. - Phospholipase A2 activation as a therapeutic approach for cognitive enhancement in early-stage Alzheimer disease.
Schaeffer EL, Forlenza OV, Gattaz WF. Schaeffer EL, et al. Psychopharmacology (Berl). 2009 Jan;202(1-3):37-51. doi: 10.1007/s00213-008-1351-0. Epub 2008 Oct 14. Psychopharmacology (Berl). 2009. PMID: 18853146 Review. - PARP Inhibition Prevents Ethanol-Induced Neuroinflammatory Signaling and Neurodegeneration in Rat Adult-Age Brain Slice Cultures.
Tajuddin N, Kim HY, Collins MA. Tajuddin N, et al. J Pharmacol Exp Ther. 2018 Apr;365(1):117-126. doi: 10.1124/jpet.117.245290. Epub 2018 Jan 16. J Pharmacol Exp Ther. 2018. PMID: 29339456 Free PMC article. - Neuroinflammation and neurodegeneration in adult rat brain from binge ethanol exposure: abrogation by docosahexaenoic acid.
Tajuddin N, Moon KH, Marshall SA, Nixon K, Neafsey EJ, Kim HY, Collins MA. Tajuddin N, et al. PLoS One. 2014 Jul 16;9(7):e101223. doi: 10.1371/journal.pone.0101223. eCollection 2014. PLoS One. 2014. PMID: 25029343 Free PMC article. - Low-dose aspirin (acetylsalicylate) prevents increases in brain PGE2, 15-epi-lipoxin A4 and 8-isoprostane concentrations in 9 month-old HIV-1 transgenic rats, a model for HIV-1 associated neurocognitive disorders.
Blanchard HC, Taha AY, Rapoport SI, Yuan ZX. Blanchard HC, et al. Prostaglandins Leukot Essent Fatty Acids. 2015 May;96:25-30. doi: 10.1016/j.plefa.2015.01.002. Epub 2015 Jan 13. Prostaglandins Leukot Essent Fatty Acids. 2015. PMID: 25638779 Free PMC article.
References
- Griffin WS. Inflammation and neurodegenerative diseases. Am J Clin Nutr. 2006;83:470S–474S. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources