Simulating properties of in vitro epithelial cell morphogenesis - PubMed (original) (raw)
Simulating properties of in vitro epithelial cell morphogenesis
Mark R Grant et al. PLoS Comput Biol. 2006.
Abstract
How do individual epithelial cells (ECs) organize into multicellular structures? ECs are studied in vitro to help answer that question. Characteristic growth features include stable cyst formation in embedded culture, inverted cyst formation in suspension culture, and lumen formation in overlay culture. Formation of these characteristic structures is believed to be a consequence of an intrinsic program of differentiation and de-differentiation. To help discover how such a program may function, we developed an in silico analogue in which space, events, and time are discretized. Software agents and objects represent cells and components of the environment. "Cells" act independently. The "program" governing their behavior is embedded within each in the form of axioms and an inflexible decisional process. Relationships between the axioms and recognized cell functions are specified. Interactions between "cells" and environment components during simulation give rise to a complex in silico phenotype characterized by context-dependent structures that mimic counterparts observed in four different in vitro culture conditions: a targeted set of in vitro phenotypic attributes was matched by in silico attributes. However, for a particular growth condition, the analogue failed to exhibit behaviors characteristic of functionally polarized ECs. We solved this problem by following an iterative refinement method that improved the first analogue and led to a second: it exhibited characteristic differentiation and growth properties in all simulated growth conditions. It is the first model to simultaneously provide a representation of nonpolarized and structurally polarized cell types, and a mechanism for their interconversion. The second analogue also uses an inflexible axiomatic program. When specific axioms are relaxed, growths strikingly characteristic of cancerous and precancerous lesions are observed. In one case, the simulated cause is aberrant matrix production. Analogue design facilitates gaining deeper insight into such phenomena by making it easy to replace low-resolution components with increasingly detailed and realistic components.
Conflict of interest statement
Competing interests. CAH is a Trustee of the CDH Research Foundation.
Figures
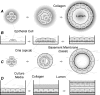
Figure 1. EC Growth Characteristics in Four Different In Vitro Culture Conditions (Cross-Section)
For each culture condition, development typically starts with one or several ECs. (A) Cell division, apoptosis, and shape change over a period of several days in embedded culture often leads to a stable, lumen-containing cyst formed by a single layer of ECs. (B) ECs plated on a layer of collagen (surface culture) typically generate a stable, uniform monolayer. (C) Inverted cysts (cell polarity relative to that in (A) is inverted) form in suspension culture, with matrix deposited on the inside of the cyst. (D) Lumens are frequently formed in collagen overlay experiments.
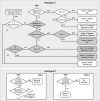
Figure 2. A Process for Iterative Refinement of Biological Models
An abstract Venn-like diagram illustrates the behavior similarities (depicted as overlapping sets of features) of in silico and in vitro models. (A) The shaded circle contains the set of observable, measurable attributes (qualities, properties, etc.) of an in silico model such as Analogue 1 or 2. The larger circle is the infinite set of possible observable, measurable attributes of an in vitro model that is being viewed or studied from a particular perspective, with attention focused on selected aspects of the system. a: Each small shaded domain represents the results of wet-lab experiments intended to measure a specific in vitro property or characteristic. The degree of shading illustrates different levels of experimental and measurement uncertainty. t: a member of the set of targeted in vitro attributes that the in silico model is expected to mimic. (B) This sketch illustrates that it may take many different in silico models, possibly drawn from different classes of models, to obtain even partial behavior coverage of the in vitro system. (C) Illustrated is the systematic, sequential extension of the in silico analogue's measurable attributes to improve coverage. The original shaded set can illustrate Analogue 1. The first dashed circle illustrates expanding the original set of targeted attributes by including one or more additional properties. The established analogue (Analogue 1) fails to generate the required outcomes, resulting in its invalidation. A copy of the established analogue can be iteratively refined (without losing or breaking the original behaviors) by adding new details as needed. The goal is to have all of the expanded set of targeted attributes (original plus expanded coverage) adequately represented by a new analogue, such as Analogue 2, represented by the first dashed circle. The process can be repeated indefinitely by adding attributes to the target set and then adding new capabilities to Analogue 2 as before. With each extension, the goal is to have the expanded set of targeted attributes all adequately represented. If the goal cannot be achieved without “breaking” the original model, then either a new model or multiple separate models, as in (B), may be required.
Figure 3. Axioms Developed from In Vitro Observations of EC Behavior in Different Culture Conditions
They specify the action a
cell
will take given a precondition: the composition and relative arrangement of components in its local neighborhood, directly adjacent to the
cell
. The neighborhood can contain one, two, or three types of object (
cell, matrix,
and
free space
). Black grid spaces represent
free space
; circular, shaded spheres represent
cells;
and white grid spaces represent
matrix
. When multiple neighborhood arrangements meet the requirements for placement of a daughter
cell
, one is selected at random. If a particular environment configuration does not meet one of the listed conditions for division or death, the
cell
does nothing.
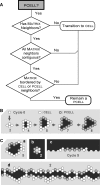
Figure 4. The Flow of Decisionmaking—Axiom Application—by Each Cell During Simulation
During each simulation cycle, each
cell
has an option to act (the order of selection is randomized each cycle). For Analogue 1 the basic options are
die, divide,
move, and add
matrix
, or do nothing. Analogue 2 (lower boxes) provides three additional options: transition to
pcell
, remain a
pcell
, or transition to
cell
status. When its turn arrives, the
cell
assesses the status of its local neighborhood (types of components and their relative locations). That information establishes the precondition for axiom application. The diagram abstracts up from many different particular simulation runs of the analogue and represents possible chains of inference that any one
cell
may follow in any given simulation cycle. For each precondition, one axiom applies and one action results. During the next simulation cycle, the process repeats, independent of what occurred in any prior cycle.
Figure 5. Examples of Analogue 1 Outcomes in Four Simulated Environments
Spheres represent
cells
; black space represents
free space
or a simulated lumen space; a light shaded space represents
matrix
. (A) Stable
cysts
formed in simulated embedded culture. (B) A stable, inverted
cyst
formed in simulated suspension culture. (C) A stable
monolayer
formed in simulated surface culture. (D) A stable
lumen
formed in simulated overlay culture.
Figure 6. An Invalid Response from Analogue 1
The sketched screenshots show the induced growth response of a monolayer of
cells
in simulated surface culture following placement of a second layer of
cells
above the first. A black space represents
free space
or a simulated lumen space; a light shaded space represents
matrix
. All the cells in a stable monolayer in vitro are polarized and so are expected to be relatively unresponsive to the newly added cells. First, a stable monolayer of
cells
was formed. The simulation was stopped. A layer of
cells
was placed on top of the monolayer (top: cycle 0). The simulation was restarted. End of cycle 1: many of the added
cells
have “died;” growth and development of
cells
in the original monolayer have been stimulated; encroachment of
cells
into the
matrix
below has started. Cycle 2: remodeling is under way. Cycle 15: after considerable remodeling, a stable structure along with several inverted
cysts
has formed.
Figure 7. Analogue 2: Preconditions Determining Transitions between Cell and Pcell, or Lack Thereof
The decisional sequences followed during simulation are illustrated in Figures 4 and 8. (A) During a simulation cycle each
cell
assesses its neighborhood and consults the axioms to determine if it qualifies to become a
pcell
(Figure 4, lower left). Any one of the three arrangements shown qualifies. If qualified, the transition occurs immediately and it enters the next simulation cycle as a
pcell
. (B) During a simulation cycle each
pcell
(that was a
pcell
in the preceding simulation cycle) assesses its neighborhood and consults the axioms to determine if it qualifies to stay a
pcell
(Figure 4, lower right). The three arrangements shown (middle column) do not qualify. If not qualified (to remain a
pcell
), the transition to
cell
occurs immediately. (C) Illustrated are changes to a
pcell'
s local neighborhood that do not meet any of the preconditions for a
pcell-
to-
cell
transition; it remains a
pcell
.
Figure 8. Retention of Pcell Status
(A) Given a
pcell
precondition, if three conditions in sequence are also met, as illustrated by the decisional flow diagram, then
pcell
status is retained. Otherwise, the
pcell
transitions to
cell
. (B) Sketched are sequential screen shots taken during a typical Analogue 2 simulation for simulated embedded culture. Spheres represent
cells
and
pcells; pcells
are shaded darker. A black space represents
free space
or a simulated lumen space; a light shaded space represents
matrix
. The first evidence of
pcells
is at the end of simulation cycle 5. Cycle 8: a stable structure has formed; only
pcells
are present. (C) An example of an Analogue 2 outcome from each of the four simulated environments, comparable to the Analogue 1 outcome in Figure 5. The status of the developing structures in the four simulated environments at simulation cycle 2 or 5 is shown. Each contains a mixture of
cells
and
pcells
: a: a developing
cyst
in simulated embedded culture; b: a
cyst
in simulated suspension culture; c:
monolayer
formation in simulated surface culture; and d:
lumen
formation in simulated overlay culture.
Figure 9. An Appropriate Response by Analogue 2
An appropriate response is demonstrated by Analogue 2 in the previously invalidating experiment described in Figure 6. This experiment determined that inclusion of
pcells
in Analogue 2 provides some resistance to the unacceptable, dramatic behavior of Analogue 1. Sketched are screen shots taken during a typical simulation.
Pcells
are shaded darker than
cells
. Spaces are shaded as defined in Figure 8. First, a stable monolayer of
pcells
was formed. The simulation was stopped. A layer of
cells
was placed on top of the
pcell
monolayer (top: cycle 0). The simulation was restarted. End of cycle 1: many of the added
cells
have “died,” and a few of the original
pcells
have transitioned to
cell
status. Cycle 5: some minor remodeling of the original layer of
matrix
has occurred; compare with cycle 2 in Figure 6. Cycle 15: a stable structure has formed dominated by
pcells;
some inverted
cysts
remain above the
pcell
monolayer.
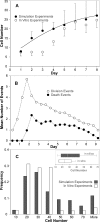
Figure 10. Analogue 2: Comparisons of In Silico and In Vitro Results
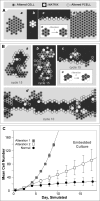
Comparisons are presented of data from experiments in silico (n = 50), using Analogue 2, and in vitro data. A mean
cell
division time of 12.0 h is assumed. (A)
Cell
numbers per
cyst
in simulated embedded culture. In vitro cell number per cyst data was adapted from the cyst size time series in embedded culture provided in Figure 9 in [7], assuming an EC diameter of 10 μm and spherical hollow cysts. The bars indicate one standard error of the mean. (B) Average division event and death event counts at each simulation cycle. (C) The frequency distribution of cell numbers in vitro per cyst cross section after ten days in embedded culture are compared withthe frequency distribution of
cell
numbers per
cyst
cross section after 12 simulation cycles; black bars: simulation results; white bars: in vitro measurements. The former (in vitro) dataset was obtained by measuring MDCK cyst sizes at day 10 from Figure 2A in [24] (n = 44), assuming an EC diameter of 10 μm and spherical hollow cysts. The latter dataset was the result of n = 1,000 simulations. The insert shows the box plots for both datasets. The box centerline designates the median; the box designates the first through third quartiles; and the whiskers represent the 10% and 90% quantiles.
Figure 11. Consequence of Altering Two Axioms, 1 and 5
Growth characteristics that are a consequence of altering two axioms (1 and 5) controlling death and division are illustrated for four simulated culture conditions. As shown at the top in (A) and (B),
cells
have different shading than they do in Figures 8 and 9 because their behaviors are governed by a different set of axioms. Spaces are shaded as defined in Figure 8. The behavior of a
pcell
, when formed, was not influenced by the altered axioms. (A) Growth resulting from blocking
cell
death in Axioms 1 and 5 (Alteration 1). (B) Growth resulting from a simulated loss on an aspect of polarity caused by disrupting the directional cell placement in Axioms 7 and 8 so that daughter cells would be placed in any free space (Alteration 2). In both (A) and (B) the simulated growth conditions are as follows; a: simulated embedded culture; b: simulated suspension culture; c: simulated surface culture; and d: simulated overlay culture. In both (A) and (B) an example of altered axioms is provided by the insert e. (C)
Cell
and
pcell
growth is plotted for simulations in embedded culture that use either the normal axioms (Figures 3 and 7) or follow Alteration 1 or 2. The vertical bars are ± one standard deviation for 50 independent simulations.
Figure 12. Consequences of Altered Matrix Production
Altered matrix production causes loss of growth control in simulated culture conditions. Axioms 4 and 5 were replaced with a new one (an example of which is shown in the insert) that allowed for de novo
matrix
production in any environment consisting of
free space
and
cell
neighbors (Alteration 3). This sequence of illustrations of typical simulation outcomes shows the consequences of Alteration 3 on
cyst
formation in simulated embedded culture. The behavior of a
pcell
, when formed, was not influenced by the altered axiom, only the behavior of
cells.
As in Figure 11,
cell
shading is different to identify that an altered axiom set was used. Spaces are shaded as defined in Figure 8. At simulation cycle 0 a
cell
is added to a
matrix
-filled grid. At the conclusion of cycle 4, a small cluster had formed containing two
pcells.
By cycle 8 a distinct pattern of unstable growth was evident, with
matrix-
and
free space
–containing,
lumen
-like spaces within clusters of
cells
. That trend was still evident at cycle 16; the outer edge contained a few
pcells
. Thereafter, there was no growth.
Similar articles
- Simulating in vitro epithelial morphogenesis in multiple environments.
Grant MR, Kim SH, Hunt CA. Grant MR, et al. Comput Syst Bioinformatics Conf. 2006:381-4. Comput Syst Bioinformatics Conf. 2006. PMID: 17369657 - Polarized epithelial cysts in vitro: a review of cell and explant culture systems that exhibit epithelial cyst formation.
McAteer JA, Dougherty GS, Gardner KD Jr, Evan AP. McAteer JA, et al. Scanning Microsc. 1988 Sep;2(3):1739-63. Scanning Microsc. 1988. PMID: 3059485 Review. - Computational investigation of epithelial cell dynamic phenotype in vitro.
Kim SH, Park S, Mostov K, Debnath J, Hunt CA. Kim SH, et al. Theor Biol Med Model. 2009 May 28;6:8. doi: 10.1186/1742-4682-6-8. Theor Biol Med Model. 2009. PMID: 19476639 Free PMC article. - Opinion: Building epithelial architecture: insights from three-dimensional culture models.
O'Brien LE, Zegers MM, Mostov KE. O'Brien LE, et al. Nat Rev Mol Cell Biol. 2002 Jul;3(7):531-7. doi: 10.1038/nrm859. Nat Rev Mol Cell Biol. 2002. PMID: 12094219 Review. - Culture requirements of prostatic epithelial cell lines for acinar morphogenesis and lumen formation in vitro: role of extracellular calcium.
Tyson DR, Inokuchi J, Tsunoda T, Lau A, Ornstein DK. Tyson DR, et al. Prostate. 2007 Nov 1;67(15):1601-13. doi: 10.1002/pros.20628. Prostate. 2007. PMID: 17705248
Cited by
- Predicting and elucidating the post-printing behavior of 3D printed cancer cells in hydrogel structures by integrating in-vitro and in-silico experiments.
Mohammadrezaei D, Moghimi N, Vandvajdi S, Powathil G, Hamis S, Kohandel M. Mohammadrezaei D, et al. Sci Rep. 2023 Jan 21;13(1):1211. doi: 10.1038/s41598-023-28286-9. Sci Rep. 2023. PMID: 36681762 Free PMC article. - MDCK cystogenesis driven by cell stabilization within computational analogues.
Engelberg JA, Datta A, Mostov KE, Hunt CA. Engelberg JA, et al. PLoS Comput Biol. 2011 Apr;7(4):e1002030. doi: 10.1371/journal.pcbi.1002030. Epub 2011 Apr 7. PLoS Comput Biol. 2011. PMID: 21490722 Free PMC article. - From cells to organs: building polarized tissue.
Bryant DM, Mostov KE. Bryant DM, et al. Nat Rev Mol Cell Biol. 2008 Nov;9(11):887-901. doi: 10.1038/nrm2523. Nat Rev Mol Cell Biol. 2008. PMID: 18946477 Free PMC article. Review. - Translatability and transferability of in silico models: Context of use switching to predict the effects of environmental chemicals on the immune system.
Pappalardo F, Russo G, Corsini E, Paini A, Worth A. Pappalardo F, et al. Comput Struct Biotechnol J. 2022 Mar 26;20:1764-1777. doi: 10.1016/j.csbj.2022.03.024. eCollection 2022. Comput Struct Biotechnol J. 2022. PMID: 35495116 Free PMC article. - Essential operating principles for tumor spheroid growth.
Engelberg JA, Ropella GE, Hunt CA. Engelberg JA, et al. BMC Syst Biol. 2008 Dec 23;2:110. doi: 10.1186/1752-0509-2-110. BMC Syst Biol. 2008. PMID: 19105850 Free PMC article.
References
- O'Brien LE, Zegers MM, Mostov KE. Opinion: Building epithelial architecture: Insights from three-dimensional culture models. Nat Rev Mol Cell Biol. 2002;3:531–537. - PubMed
- Walker DC, Hill G, Wood SM, Smallwood RH, Southgate J. Agent-based computational modeling of wounded epithelial cell monolayers. IEEE Trans Nanobioscience. 2004;3:153–163. - PubMed
- Brenner S. Biological computation. In: Bock GR, editor. The limits of reductionism in biology. Chichester (United Kingdom): Wiley; 1998. pp. 106–116.
- Noble D. The future: Putting Humpty-Dumpty together again. Biochem Soc Trans. 2003;31:156–158. - PubMed