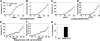VEGF-C-induced lymphangiogenesis in sentinel lymph nodes promotes tumor metastasis to distant sites - PubMed (original) (raw)
VEGF-C-induced lymphangiogenesis in sentinel lymph nodes promotes tumor metastasis to distant sites
Satoshi Hirakawa et al. Blood. 2007.
Abstract
The mechanisms by which tumors metastasize to sentinel and distant lymph nodes, and beyond, are poorly understood. We developed transgenic mice that overexpress vascular endothelial growth factor-C (VEGF-C) and green fluorescent protein specifically in the skin and studied the effects of chemically-induced skin carcinogenesis in this model. We found that in contrast to VEGF-A, VEGF-C does not increase the growth of primary tumors, but instead induces expansion of lymphatic networks within sentinel lymph nodes, even before the onset of metastasis. Once the metastatic cells arrived at the sentinel lymph nodes, the extent of lymphangiogenesis at these sites increased. Of importance, in mice with metastasis-containing sentinel lymph nodes, tumors that expressed VEGF-C were more likely to metastasize to additional organs, such as distal lymph nodes and lungs. No metastases were observed in distant organs in the absence of lymph node metastases. These findings indicate an important role of VEGF-C-induced lymph node lymphangiogenesis in the promotion of cancer metastasis beyond the sentinel lymph nodes. VEGF-C is therefore a good target to slow or even prevent the onset of metastasis.
Figures
Figure 1
Transgenic overexpression of VEGF-C does not affect the number or frequency of papillomas and squamous-cell carcinomas that form in mice. (A) Incidence of skin papilloma formation, over time (weeks), in VEGF-C transgenic mice (n = 31; ■), compared with control mice (n = 32; ○). Incidence is expressed as the percentage of mice with detectable papillomas (> 1 mm) during the 20 weeks of topical PMA application. (B) No significant increase in the frequency (average number of papillomas per mouse) of papilloma formation was observed in VEGF-C transgenic mice. (C-D) No differences were observed in incidence or number of large papillomas more than 3 mm that formed in VEGF-C transgenic mice, compared with control mice, over the 20-week period of carcinogen application. (E-F) When mice were observed for an extended time period (33 weeks), squamous-cell carcinomas (SCCs) developed in both control and VEGF-C transgenic mice with the same incidence and numbers. (G) A comparable percentage of large papillomas underwent malignant conversion into SCCs in VEGF-C transgenic mice and control mice.
Figure 2
Transgenic VEGF-C expression is maintained during skin carcinogenesis. In situ hybridization demonstrates strong expression of human VEGFC mRNA in squamous-cell carcinomas (SCCs) of VEGF-C transgenic mice. (A) In primary tumors, SCC cells express high levels of VEGFC mRNA, as indicated by hybridization to an antisense human VEGFC probe. (B) In SCC metastases that form in sentinel lymph nodes (LNs), this expression of VEGF-C mRNA is maintained. (C-D) A human VEGF-C sense control probe did not hybridize with tumor samples. (A-D) Scale bars represent 100 μm. (E) ELISA analysis of human VEGF-C protein expression in skin and tumor lysates (n = 5 per group) revealed significant increases in the levels of VEGF-C protein in SCCs that form in VEGF-C transgenic mice, compared with the normal skin of these mice. No human VEGF was detected in skin or tumor lysates of control mice. Data are expressed as mean ± SD; ***P < .001.
Figure 3
Increased tumor lymphangiogenesis and angiogenesis in VEGF-C transgenic mice. Immunofluorescence analyses of CD31 (green) and LYVE-1 (red) expression in normal skin (A-B), early papillomas (C-D), and SCCs (E-F) of control (WT; A,C,E) and VEGF-C transgenic (VEGFC TG; B,D,F) mice revealed increased vascularization of papillomas and SCCs in VEGF-C transgenic mice and in control mice, compared with their PMA-treated skin. Tumor lymphangiogenesis was more prominent in VEGFC transgenic mice (D,F) than in control mice (C,E), with increased numbers of enlarged lymphatic vessels (red). Slight increases in the amount of tumor angiogenesis (green) in SCCs of VEGFC transgenic mice (F) were also observed, compared with that of control mice (E). Nuclei are labeled blue (Hoechst stain). (A-F) Scale bars represent 200 μm. (G-H) Computer-assisted morphometric analysis of normal cutaneous vessels and of tumor-associated lymphatic and blood vessels was performed. A significant increase in the relative area occupied by blood vessels in the peritumoral area of SCCs (Peri SCC), as well as within SCCs (Intra SCC), was observed in VEGFC transgenic mice (TG; ■), compared with that of the control mice (WT, □) (G). A significant increase of the relative area occupied by lymphatic vessels was observed in the VEGFC transgenic mice, throughout all stages of skin carcinogenesis (H). Skin indicates PMA-treated normal skin (n = 7); SP, small papillomas (1-3 mm; n = 6); LP, large papillomas (> 3 mm; n = 6); and SCC, squamous-cell carcinoma (n = 7). Data are expressed as mean ± SEM. *P < .05; **P < .01; ***P < .001; NS = not significant.
Figure 4
Increased expression of VEGFR-3 in tumor-associated lymphatic vessels of VEGFC transgenic mice. Immunofluorescence analysis of LYVE-1 (red) and VEGFR-3 (green) expression was performed in SCC samples from control (A,C,E) and VEGFC transgenic (TG; B,D,F) mice. Based on LYVE-1 expression, the SCCs of VEGFC transgenic mice demonstrated prominent lymphangiogenesis (B), whereas tumor-associated lymphatic vessels were less pronounced in SCCs of control mice (A). Expression of the VEGF-C receptor (VEGFR-3) was strongly up-regulated in the tumor-associated lymphatic vessels of VEGF-C–overexpressing mice (D). Merging of images revealed that VEGFR-3 expression completely colocalized with that of LYVE-1 (F, yellow to orange), whereas VEGFR-3 was only weakly expressed in the tumor-associated lymphatic vessels of control mice (C,E). (A-F) Scale bars represent 200 μm.
Figure 5
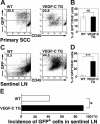
Increased tumor metastasis to sentinel lymph nodes in VEGFC transgenic mice. (A-D) Flow cytometry was used to calculate the percentage of GFP-expressing tumor cells in primary squamous-cell carcinomas (SCC; A-B) and in sentinel lymph nodes (LN; C-D) of control and VEGFC transgenic mice. Of all SCC-associated cells, 25.5% in the control GFP-transgenic mice and 23.8% in the VEGFC transgenic mice were observed to be GFP positive (A-B). Eight weeks after the first cutaneous SCCs were detected, the number and percentage of GFP-expressing tumor cells was significantly higher in metastases that formed in the sentinel lymph nodes of the VEGFC transgenic mice (44.5%) than of the control GFP-transgenic mice (0.3%; C). Data are expressed as mean ± SEM (n = 5 per group). (E) Fluorescence microscopy analysis revealed an increased incidence of sentinel lymph node metastasis in VEGFC transgenic mice, compared with control mice (n = 12). (B,D,E) ***P < .001; *P < .05; NS = no significance.
Figure 6
Prominent lymph node lymphangiogenesis in VEGFC transgenic mice. (A-B) Routine H&E stains of lymph nodes of non–tumor-bearing mice. (C-D) Double immunofluorescence staining of lymph nodes of non–tumor-bearing mice demonstrated a comparable pattern of CD31+/LYVE-1–negative blood vessels (green) and LYVE-1–positive sinusoids (red) in wild-type mice (C) and in VEGFC transgenic (TG; D) mice. Nonmetastatic sentinel lymph nodes of SCC-bearing VEGFC transgenic mice have increased numbers of enlarged LYVE-1–positive sinusoids (red; F), compared with control mice (E). An increased number of enlarged LYVE-1–positive lymphatic vessels (red) was also found in the metastatic sentinel lymph nodes of VEGFC transgenic mice (H), compared with control mice (G). The tumor-associated LYVE-1–positive vessels (red) in VEGFC transgenic mice showed high levels of BrdU staining in lymphatic endothelial cells (J; green), indicating active lymphatic proliferation (arrowheads) within sentinel lymph nodes. These cells also expressed Prox1 (I; green). Nuclei are stained blue (Hoechst stain). (A-F, I-J) Scale bars represent 100 μm; (G-H) scale bars represent 200 μm.
Similar articles
- VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis.
Hirakawa S, Kodama S, Kunstfeld R, Kajiya K, Brown LF, Detmar M. Hirakawa S, et al. J Exp Med. 2005 Apr 4;201(7):1089-99. doi: 10.1084/jem.20041896. J Exp Med. 2005. PMID: 15809353 Free PMC article. - Induced lymphatic sinus hyperplasia in sentinel lymph nodes by VEGF-C as the earliest premetastatic indicator.
Liersch R, Hirakawa S, Berdel WE, Mesters RM, Detmar M. Liersch R, et al. Int J Oncol. 2012 Dec;41(6):2073-8. doi: 10.3892/ijo.2012.1665. Epub 2012 Oct 16. Int J Oncol. 2012. PMID: 23076721 Free PMC article. - Tumor-secreted vascular endothelial growth factor-C is necessary for prostate cancer lymphangiogenesis, but lymphangiogenesis is unnecessary for lymph node metastasis.
Wong SY, Haack H, Crowley D, Barry M, Bronson RT, Hynes RO. Wong SY, et al. Cancer Res. 2005 Nov 1;65(21):9789-98. doi: 10.1158/0008-5472.CAN-05-0901. Cancer Res. 2005. PMID: 16267000 - Lymphangiogenesis and tumor metastasis.
Nisato RE, Tille JC, Pepper MS. Nisato RE, et al. Thromb Haemost. 2003 Oct;90(4):591-7. doi: 10.1160/TH03-04-0206. Thromb Haemost. 2003. PMID: 14515178 Review. - From tumor lymphangiogenesis to lymphvascular niche.
Hirakawa S. Hirakawa S. Cancer Sci. 2009 Jun;100(6):983-9. doi: 10.1111/j.1349-7006.2009.01142.x. Epub 2009 Feb 20. Cancer Sci. 2009. PMID: 19385973 Free PMC article. Review.
Cited by
- Pre-metastatic niche: formation, characteristics and therapeutic implication.
Wang Y, Jia J, Wang F, Fang Y, Yang Y, Zhou Q, Yuan W, Gu X, Hu J, Yang S. Wang Y, et al. Signal Transduct Target Ther. 2024 Sep 25;9(1):236. doi: 10.1038/s41392-024-01937-7. Signal Transduct Target Ther. 2024. PMID: 39317708 Free PMC article. Review. - Exosomal miRNAs: the tumor's trojan horse in selective metastasis.
Bayat M, Sadri Nahand J. Bayat M, et al. Mol Cancer. 2024 Aug 20;23(1):167. doi: 10.1186/s12943-024-02081-0. Mol Cancer. 2024. PMID: 39164756 Free PMC article. Review. - UBE2C-induced crosstalk between mono- and polyubiquitination of SNAT2 promotes lymphatic metastasis in bladder cancer.
Li W, Chen C, Zheng H, Lin Y, An M, Liu D, Zhang Y, Gao M, Lan T, He W. Li W, et al. J Clin Invest. 2024 Jul 1;134(13):e179122. doi: 10.1172/JCI179122. J Clin Invest. 2024. PMID: 38949026 Free PMC article. - The Role of Salivary Vascular Endothelial Growth Factor A, Cytokines, and Amino Acids in Immunomodulation and Angiogenesis in Breast Cancer.
Sarf EA, Dyachenko EI, Bel'skaya LV. Sarf EA, et al. Biomedicines. 2024 Jun 14;12(6):1329. doi: 10.3390/biomedicines12061329. Biomedicines. 2024. PMID: 38927536 Free PMC article. - Extracellular vesicles and particles as mediators of long-range communication in cancer: connecting biological function to clinical applications.
Asao T, Tobias GC, Lucotti S, Jones DR, Matei I, Lyden D. Asao T, et al. Extracell Vesicles Circ Nucl Acids. 2023 Sep;4(3):461-485. doi: 10.20517/evcna.2023.37. Epub 2023 Aug 16. Extracell Vesicles Circ Nucl Acids. 2023. PMID: 38707985 Free PMC article.
References
- Oliver G, Detmar M. The rediscovery of the lymphatic system: old and new insights into the development and biological function of the lymphatic vasculature. Genes Dev. 2002;16:773–783. - PubMed
- Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005;438:946–953. - PubMed
- Prevo R, Banerji S, Ferguson DJ, Clasper S, Jackson DG. Mouse LYVE-1 is an endocytic receptor for hyaluronan in lymphatic endothelium. J Biol Chem. 2001;276:19420–19430. - PubMed
- Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–778. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA86410/CA/NCI NIH HHS/United States
- R01 CA069184/CA/NCI NIH HHS/United States
- P01 CA092644/CA/NCI NIH HHS/United States
- R01 HL075183/HL/NHLBI NIH HHS/United States
- CA92644/CA/NCI NIH HHS/United States
- CA69184/CA/NCI NIH HHS/United States
- R01 CA086410/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases