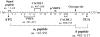Mutation in the auxiliary calcium-channel subunit CACNA2D4 causes autosomal recessive cone dystrophy - PubMed (original) (raw)
Case Reports
Mutation in the auxiliary calcium-channel subunit CACNA2D4 causes autosomal recessive cone dystrophy
Katharina Agnes Wycisk et al. Am J Hum Genet. 2006 Nov.
Abstract
Retinal signal transmission depends on the activity of high voltage-gated l-type calcium channels in photoreceptor ribbon synapses. We recently identified a truncating frameshift mutation in the Cacna2d4 gene in a spontaneous mouse mutant with profound loss of retinal signaling and an abnormal morphology of ribbon synapses in rods and cones. The Cacna2d4 gene encodes an l-type calcium-channel auxiliary subunit of the alpha (2) delta type. Mutations in its human orthologue, CACNA2D4, were not yet known to be associated with a disease. We performed mutation analyses of 34 patients who received an initial diagnosis of night blindness, and, in two affected siblings, we detected a homozygous nucleotide substitution (c.2406C-->A) in CACNA2D4. The mutation introduces a premature stop codon that truncates one-third of the corresponding open reading frame. Both patients share symptoms of slowly progressing cone dystrophy. These findings represent the first report of a mutation in the human CACNA2D4 gene and define a novel gene defect that causes autosomal recessive cone dystrophy.
Figures
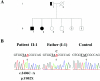
Figure 1.
Mutation analysis of CACNA2D4 of index patient II-1. A, Pedigree of the family of patient II-1. Blackened symbols indicate affected status, the half-blackened symbol indicates mutation carrier, unblackened symbols indicate unaffected status, squares represent males, and circles represent females. Sequence analysis revealed a homozygous c.2406C→A mutation in the index patient and in his affected sister (II-2) as well as the carrier status of the father (I-1). Mutation analyses of the two unaffected siblings (II-3 and II-4) displayed homozygosity for the wild-type allele. B, Electropherograms show the respective DNA sequences of exon 25 of CACNA2D4. The homozygous c.2406C→A mutation in patient II-1 (left), the heterozygous mutation in the unaffected father I-1 (middle), and the wild-type allele of a control DNA sample (right) are indicated. The position of the mutated nucleotide is indicated by an arrow.
Figure 2.
Schematic representation of CACNA2D4 transcript. The schematic drawing shows the longest transcript variant of human CACNA2D4 (GenBank accession number NM_172364.3) (fig. not to scale). Its ORF codes for a protein of 1,137 aa residues, posttranslationally cleaved into the α2 (aa 65–991; 232–3,204 bp) and the δ (aa 992–1137; 3,205–3,645 bp) peptides. The cleavage site between the α2 and δ peptides, encoded by an alanine residue at position 992 (exon 34), is indicated by an arrow. The locations of the conserved von Willebrand factor A (VWFA) domain and the calcium-channel and chemotaxis receptor (CACHE) domains are illustrated in the transcript scheme. The CACHE domains probably constitute binding sites for small ligands, whereas the VWFA domain is responsible for protein-protein interactions of the α2δ protein with α1 subunits. At the N-terminus, the CACNA2D4 protein also contains a putative signal peptide of 64 aa residues.,
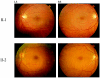
Figure 3.
Fundus photographs of patients II-1 and II-2. Fundus of patient II-1 (upper panels) and patient II-2 (lower panels) of the left eye (LE) and right eye (RE), respectively, show nearly normal appearance.
Figure 4.
ERG of patients carrying the p.Y802X mutation and ERG recordings in the right eye of patients II-1 and II-2. The left panel shows representative recordings of an unaffected subject, the middle panel shows the recordings of patient II-1, and the right panel shows recordings of patient II-2. Rod responses (A), maximal combined responses (B), oscillatory potentials (C), single-flash cone responses (D), and 30-Hz flicker responses (E), each according to ISCEV standard, are shown. The negative-oriented a-wave of an ERG response reflects the hyperpolarization of photoreceptors due to a flash of light. The positive-oriented b-wave represents a summation of responses evoked by activities of secondary neurons during photoreceptor synaptic signal transmission. Both patients share a frequency doubling of the 30-Hz flicker ERG.
Similar articles
- Structural and functional abnormalities of retinal ribbon synapses due to Cacna2d4 mutation.
Wycisk KA, Budde B, Feil S, Skosyrski S, Buzzi F, Neidhardt J, Glaus E, Nürnberg P, Ruether K, Berger W. Wycisk KA, et al. Invest Ophthalmol Vis Sci. 2006 Aug;47(8):3523-30. doi: 10.1167/iovs.06-0271. Invest Ophthalmol Vis Sci. 2006. PMID: 16877424 - A New Zebrafish Model for CACNA2D4-Dysfunction.
Schlegel DK, Glasauer SMK, Mateos JM, Barmettler G, Ziegler U, Neuhauss SCF. Schlegel DK, et al. Invest Ophthalmol Vis Sci. 2019 Dec 2;60(15):5124-5135. doi: 10.1167/iovs.19-26759. Invest Ophthalmol Vis Sci. 2019. PMID: 31834350 - A novel homozygous nonsense mutation in CABP4 causes congenital cone-rod synaptic disorder.
Littink KW, van Genderen MM, Collin RW, Roosing S, de Brouwer AP, Riemslag FC, Venselaar H, Thiadens AA, Hoyng CB, Rohrschneider K, den Hollander AI, Cremers FP, van den Born LI. Littink KW, et al. Invest Ophthalmol Vis Sci. 2009 May;50(5):2344-50. doi: 10.1167/iovs.08-2553. Epub 2008 Dec 13. Invest Ophthalmol Vis Sci. 2009. PMID: 19074807 - Progressive cone dystrophy associated with mutation in CNGB3.
Michaelides M, Aligianis IA, Ainsworth JR, Good P, Mollon JD, Maher ER, Moore AT, Hunt DM. Michaelides M, et al. Invest Ophthalmol Vis Sci. 2004 Jun;45(6):1975-82. doi: 10.1167/iovs.03-0898. Invest Ophthalmol Vis Sci. 2004. PMID: 15161866 - Genotype-phenotype correlation in a German family with a novel complex CRX mutation extending the open reading frame.
Paunescu K, Preising MN, Janke B, Wissinger B, Lorenz B. Paunescu K, et al. Ophthalmology. 2007 Jul;114(7):1348-1357.e1. doi: 10.1016/j.ophtha.2006.10.034. Epub 2007 Feb 22. Ophthalmology. 2007. PMID: 17320181 Review.
Cited by
- The Voltage-Gated Calcium Channel α2δ Subunit in Neuropathic Pain.
Guo SJ, Shi YQ, Zheng YN, Liu H, Zheng YL. Guo SJ, et al. Mol Neurobiol. 2024 Aug 13. doi: 10.1007/s12035-024-04424-w. Online ahead of print. Mol Neurobiol. 2024. PMID: 39136907 Review. - Four different gene-related cone-rod dystrophy: clinical and genetic findings in six Chinese families with diverse modes of inheritance.
Li Z, Cheng W, Zi F, Wang J, Huang X, Sheng X, Rong W. Li Z, et al. Front Genet. 2023 Nov 2;14:1157156. doi: 10.3389/fgene.2023.1157156. eCollection 2023. Front Genet. 2023. PMID: 38028590 Free PMC article. - The endoplasmic reticulum membrane protein complex subunit Emc6 is essential for rhodopsin localization and photoreceptor cell survival.
Sun K, Liu L, Jiang X, Wang H, Wang L, Yang Y, Liu W, Zhang L, Zhao X, Zhu X. Sun K, et al. Genes Dis. 2023 May 23;11(2):1035-1049. doi: 10.1016/j.gendis.2023.03.033. eCollection 2024 Mar. Genes Dis. 2023. PMID: 37692493 Free PMC article. - Identification of candidate genes for developmental colour agnosia in a single unique family.
Nijboer TCW, Hessel EVS, van Haaften GW, van Zandvoort MJ, van der Spek PJ, Troelstra C, de Kovel CGF, Koeleman BPC, van der Zwaag B, Brilstra EH, Burbach JPH. Nijboer TCW, et al. PLoS One. 2023 Sep 6;18(9):e0290013. doi: 10.1371/journal.pone.0290013. eCollection 2023. PLoS One. 2023. PMID: 37672513 Free PMC article. - Photoreceptor Ion Channels in Signaling and Disease.
Inamdar SM, Lankford CK, Baker SA. Inamdar SM, et al. Adv Exp Med Biol. 2023;1415:269-276. doi: 10.1007/978-3-031-27681-1_39. Adv Exp Med Biol. 2023. PMID: 37440044
References
Web Resources
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for CACNA2D4 [accession number NM_172364.3])
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for CACNA2D4, CACNA1F, and CABP4)
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases