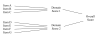Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance - PubMed (original) (raw)
Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance
U.S. Department of Health and Human Services FDA Center for Drug Evaluation and Research et al. Health Qual Life Outcomes. 2006.
Abstract
This guidance describes how the FDA evaluates patient-reported outcome (PRO) instruments used as effectiveness endpoints in clinical trials. It also describes our current thinking on how sponsors can develop and use study results measured by PRO instruments to support claims in approved product labeling (see appendix point 1). It does not address the use of PRO instruments for purposes beyond evaluation of claims made about a drug or medical product in its labeling. By explicitly addressing the review issues identified in this guidance, sponsors can increase the efficiency of their endpoint discussions with the FDA during the product development process, streamline the FDA's review of PRO endpoint adequacy, and provide optimal information about the patient's perspective of treatment benefit at the time of product approval. A PRO is a measurement of any aspect of a patient's health status that comes directly from the patient (i.e., without the interpretation of the patient's responses by a physician or anyone else). In clinical trials, a PRO instrument can be used to measure the impact of an intervention on one or more aspects of patients' health status, hereafter referred to as PRO concepts, ranging from the purely symptomatic (response of a headache) to more complex concepts (e.g., ability to carry out activities of daily living), to extremely complex concepts such as quality of life, which is widely understood to be a multidomain concept with physical, psychological, and social components. Data generated by a PRO instrument can provide evidence of a treatment benefit from the patient perspective. For this data to be meaningful, however, there should be evidence that the PRO instrument effectively measures the particular concept that is studied. Generally, findings measured by PRO instruments may be used to support claims in approved product labeling if the claims are derived from adequate and well-controlled investigations that use PRO instruments that reliably and validly measure the specific concepts at issue. The glossary defines many of the terms used in this guidance. In particular, the term instrument refers to the actual questions or items contained in a questionnaire or interview schedule along with all the additional information and documentation that supports the use of these items in producing a PRO measure (e.g., interviewer training and instructions, scoring and interpretation manual). The term conceptual framework refers to how items are grouped according to subconcepts or domains (e.g., the item walking without help may be grouped with another item, walking with difficulty, within the domain of ambulation, and ambulation may be further grouped into the concept of physical ability). FDA's guidance documents, including this guidance, do not establish legally enforceable responsibilities. Instead, guidance documents describe the Agency's current thinking on a topic and should be viewed only as recommendations, unless specific regulatory or statutory requirements are cited. The use of the word should in Agency guidance documents means that something is suggested or recommended but not required. First publication of the Draft Guidance by the Food and Drug Administration--February 2006.
Figures
Figure 1
The PRO instrument development and modification process.
Figure 2
Diagram of a conceptual framework.
Comment in
- Patient-reported outcome measures in arthroplasty registries.
Rolfson O, Eresian Chenok K, Bohm E, Lübbeke A, Denissen G, Dunn J, Lyman S, Franklin P, Dunbar M, Overgaard S, Garellick G, Dawson J; Patient-Reported Outcome Measures Working Group of the International Society of Arthroplasty Registries. Rolfson O, et al. Acta Orthop. 2016 Jul;87 Suppl 1(Suppl 1):3-8. doi: 10.1080/17453674.2016.1181815. Epub 2016 May 11. Acta Orthop. 2016. PMID: 27168175 Free PMC article.
Similar articles
- Recommendations on evidence needed to support measurement equivalence between electronic and paper-based patient-reported outcome (PRO) measures: ISPOR ePRO Good Research Practices Task Force report.
Coons SJ, Gwaltney CJ, Hays RD, Lundy JJ, Sloan JA, Revicki DA, Lenderking WR, Cella D, Basch E; ISPOR ePRO Task Force. Coons SJ, et al. Value Health. 2009 Jun;12(4):419-29. doi: 10.1111/j.1524-4733.2008.00470.x. Epub 2008 Nov 11. Value Health. 2009. PMID: 19900250 - Patient-reported outcomes supporting anticancer product approvals.
Rock EP, Kennedy DL, Furness MH, Pierce WF, Pazdur R, Burke LB. Rock EP, et al. J Clin Oncol. 2007 Nov 10;25(32):5094-9. doi: 10.1200/JCO.2007.11.3803. J Clin Oncol. 2007. PMID: 17991927 Review. - Responsiveness and minimal important differences for patient reported outcomes.
Revicki DA, Cella D, Hays RD, Sloan JA, Lenderking WR, Aaronson NK. Revicki DA, et al. Health Qual Life Outcomes. 2006 Sep 27;4:70. doi: 10.1186/1477-7525-4-70. Health Qual Life Outcomes. 2006. PMID: 17005038 Free PMC article. - Patient-reported outcomes to support medical product labeling claims: FDA perspective.
Patrick DL, Burke LB, Powers JH, Scott JA, Rock EP, Dawisha S, O'Neill R, Kennedy DL. Patrick DL, et al. Value Health. 2007 Nov-Dec;10 Suppl 2:S125-37. doi: 10.1111/j.1524-4733.2007.00275.x. Value Health. 2007. PMID: 17995471 Review.
Cited by
- The Arabic medication-related burden quality of life (MRB-QoL) tool: Cross-cultural adaptation and content validation.
Al-Ebrahim SQ, Harrison J, Chen TF, Alzubaidi H, Mohammed MA. Al-Ebrahim SQ, et al. Explor Res Clin Soc Pharm. 2024 Oct 10;16:100523. doi: 10.1016/j.rcsop.2024.100523. eCollection 2024 Dec. Explor Res Clin Soc Pharm. 2024. PMID: 39498226 Free PMC article. - How to use outcomes questionnaires: pearls and pitfalls.
Malay S, Chung KC. Malay S, et al. Clin Plast Surg. 2013 Apr;40(2):261-9. doi: 10.1016/j.cps.2012.10.002. Epub 2012 Nov 26. Clin Plast Surg. 2013. PMID: 23506766 Free PMC article. Review. - Psychometric properties of FACIT-Fatigue in systemic lupus erythematosus: a pooled analysis of three phase 3 randomised, double-blind, parallel-group controlled studies (BLISS-SC, BLISS-52, BLISS-76).
Rendas-Baum R, Baranwal N, Joshi AV, Park J, Kosinski M. Rendas-Baum R, et al. J Patient Rep Outcomes. 2021 Apr 8;5(1):33. doi: 10.1186/s41687-021-00298-x. J Patient Rep Outcomes. 2021. PMID: 33830377 Free PMC article. - Psychometric properties of computerized adaptive testing for chronic obstructive pulmonary disease patient-reported outcome measurement.
Wang J, Xie Y, Feng Z, Li J. Wang J, et al. Health Qual Life Outcomes. 2024 Sep 4;22(1):73. doi: 10.1186/s12955-024-02291-6. Health Qual Life Outcomes. 2024. PMID: 39227972 Free PMC article. - Influence of the COVID-19 Outbreak on Disease Activity and Quality of Life in Inflammatory Bowel Disease Patients.
Conti C, Rosa I, Zito L, Grossi L, Efthymakis K, Neri M, Porcelli P. Conti C, et al. Front Psychiatry. 2021 Apr 22;12:664088. doi: 10.3389/fpsyt.2021.664088. eCollection 2021. Front Psychiatry. 2021. PMID: 33967864 Free PMC article.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources