Molecular basis of RNA recognition and TAP binding by the SR proteins SRp20 and 9G8 - PubMed (original) (raw)
Molecular basis of RNA recognition and TAP binding by the SR proteins SRp20 and 9G8
Yann Hargous et al. EMBO J. 2006.
Abstract
The sequence-specific RNA-binding proteins SRp20 and 9G8 are the smallest members of the serine- and arginine-rich (SR) protein family, well known for their role in splicing. They also play a role in mRNA export, in particular of histone mRNAs. We present the solution structures of the free 9G8 and SRp20 RNA recognition motifs (RRMs) and of SRp20 RRM in complex with the RNA sequence 5'CAUC3'. The SRp20-RNA structure reveals that although all 4 nt are contacted by the RRM, only the 5' cytosine is primarily recognized in a specific way. This might explain the numerous consensus sequences found by SELEX (systematic evolution of ligands by exponential enrichment) for the RRM of 9G8 and SRp20. Furthermore, we identify a short arginine-rich peptide adjacent to the SRp20 and 9G8 RRMs, which does not contact RNA but is necessary and sufficient for interaction with the export factor Tip-associated protein (TAP). Together, these results provide a molecular description for mRNA and TAP recognition by SRp20 and 9G8.
Figures
Figure 1
Amino-acid sequences of 9G8, SRp20 and RBP1 and overviews of the solution structures of the RRMs for 9G8 and SRp20. (A) Amino-acid sequences of human 9G8, SRp20 and Drosophila RBP1. Only the first 100 aa are shown. The amino-acid number and the secondary structure are indicated in blue for 9G8 and black for SRp20. Amino acids in red are involved in RNA binding in the SRP20–CAUC complex. The arginines in SRp20 and 9G8, whose mutation into glutamate abolishes TAP binding, are highlighted in green. (B) Overlay of the final 20 structures of 9G8 RRM superposed on the backbone atoms of the structured parts of the protein. The protein backbone is red. Only the ordered region of the protein (aa 10–82) is shown. (C) The lowest energy structure in ribbon (protein backbone) and stick representation. Important protein side chains interacting with RNA in the complex are represented as green sticks. (D) Surface representation of the 9G8 RRM, indicating residues interacting with RNA by homology with the SRp20:RNA complex. (E) Overlay of the final 19 structures of SRp20 RRM superposed on the backbone atoms of the structured parts of the protein. The protein backbone is gray. Only the ordered region of the protein (aa 9–81) is shown. (F) A representative conformer of SRp20 in ribbon (protein backbone) and stick representation. Important protein side chains interacting with RNA in the complex are represented as green sticks. (G) Surface representation of the SRp20 RRM, indicating residues interacting with RNA by homology with the SRp20:RNA complex.
Figure 2
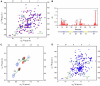
RNA containing CAUC binds to the RRM of SRp20 and 9G8 and affects residues in the β-sheet. (A) 15N HSQC spectra of ∼1 mM solutions of free GB1-SRp20 RRM (red) and that bound in the presence of one equivalent of 5′UCAUC3′ (blue) at 315 K. (B) The changes in chemical shifts of the backbone amide nitrogens and protons between free and bound RRM are plotted versus the amino-acid number. Large chemical shift changes occur in the β-strands as well as in the region immediately after β4 (X represents the position of proline residues). (C) Sections of 2D TOCSY spectra showing the H5–H6 correlations of C1, U2 and C4 for ∼1 mM solutions of free 5′CAUC3′ (red) and of 5′CAUC3′ in the presence of one-fifth (black) and one equivalent of protein (blue). (D) 15N HSQC spectra for ∼1 mM solutions of free GB1-9G8 RRM (red) and that bound in the presence of one equivalent of 5′CUCUUCAC3′ (blue) at 315 K.
Figure 3
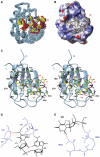
Overview of the solution structure of the SRp20 RRM in complex with CAUC. (A) Overlay of the final 29 structures superposed on the heavy atoms of the structured parts of the protein and RNA. The protein backbone is shown in cyan and RNA heavy atoms are shown in yellow (C), red (O) and blue (N and P). Only the ordered region of the RRM (residues 9–83) is shown. (B) Surface representation for the RRM (residues 9–83) and stick (RNA heavy atoms) representation for the RNA of the most representative structure of the complex. The protein surface is painted according to surface potential, with red indicating negative and blue indicating positive charges. The RNA is colored as in (A). (C) The most representative structure of the complex in ribbon (protein backbone) and stick (RNA) representation. The color scheme is the same as in (A), and important protein side chains involved in interactions with the RNA are represented as green sticks. Putative hydrogen bonds are shown by dotted violet lines. Schematic representations of the intermolecular hydrogen-bond interactions stabilizing C1 and A2 (D), and U3 (E). The RNA is shown in black and the protein in blue.
Figure 4
Identification of a TAP-binding motif in SRp20 and 9G8. (A) Schematic of full-length SRp20, deletion and point mutations used together with the amino-acid sequence for the linker (L) domain. (B) Pull-down assays. GST (control, lane −) and GST-TAP-p15 (lanes 1–6) expressed in E. coli were first immobilized on glutathione-coated beads. Various 35S-radiolabeled SRp20 proteins synthesized in rabbit reticulocytes were added to the binding reactions in the presence of ribonuclease A. Eluted proteins were analyzed following SDS–PAGE by Phosphorimaging (left, middle panels) and Coomassie blue (right panel). (C) Pull-down assays. Lanes 1 and 2 show purified proteins. Recombinant GST (control, lane 3) and GST fusions of SRp20 aa 1–90 (lane 4) or of SRp20 aa 84–90 (lane 5) were immobilized on glutathione beads and purified TAP-p15 expressed in E. coli was added to the reactions. Eluted proteins were analyzed by SDS–PAGE stained with Coomassie blue. (D) Schematic of full-length 9G8, deletion and point arginine mutations used together with the amino-acid sequence for the linker (L) domain. (E) Pull-down assays. GST (control, lane −) and GST-TAP-p15 (lanes 1–8) expressed in E. coli were first immobilized on glutathione-coated beads. Various 35S-radiolabeled 9G8 proteins synthesized in rabbit reticulocytes were added to the binding reactions in the presence of ribonuclease. Eluted proteins were analyzed following SDS–PAGE by Phosphorimaging (left, middle panels) and Coomassie blue staining (right panel). (F) Pull-down assays. Lanes 1 and 2 show purified proteins. Recombinant GST (control, lane 3) and GST fusions of 9G8 aa 12–98 (lane 4) or of 9G8 aa 81–98 (lane 5) were immobilized on glutathione beads and purified TAP-p15 expressed in E. coli was added to the reactions. Eluted proteins were analyzed by SDS–PAGE stained with Coomassie blue. Amino acids encoded by constructs used in all pull-down assays are shown in brackets after the protein name.
Figure 5
Functional analysis of the TAP-binding motif. (A) Co-immunoprecipitation assays. Total extracts from 293T cells (Mock) co-transfected with a Myc-tagged TAP plasmid and either a control (Flag) or various Flag-Myc-tagged SRp20 constructs were treated with ribonuclease A and alkaline phosphatase before immunoprecipitation with α-Flag antibodies. Total extracts (left panel) and purified complexes (right panel) were analyzed by Western immunoblotting with α-Myc antibodies. Asterisks indicate heavy and light IgG chains. (B) Schematic representation of the tethered mRNA export reporter assay. SD: splice donor; SA: splice acceptor. (C) α-Myc Western blot of total 293T extracts used for the tethered export assay. MS2 fusions of REF2-I-RRM (aa 71–155) and of the SRp20 (aa 84–90)-REF2-I-RRM chimera (chimera) reproducibly led to poor expression (shown with arrows), which can be detected only by overexposure of the blot. (D) Luciferase activity generated by the MS2 fusions in the tethered export assay. Error bars represent standard deviations from four independent sets of assays, each carried out in triplicate.
Similar articles
- Splicing factors SRp20 and 9G8 promote the nucleocytoplasmic export of mRNA.
Huang Y, Steitz JA. Huang Y, et al. Mol Cell. 2001 Apr;7(4):899-905. doi: 10.1016/s1097-2765(01)00233-7. Mol Cell. 2001. PMID: 11336712 - SR proteins SRp20 and 9G8 contribute to efficient export of herpes simplex virus 1 mRNAs.
Escudero-Paunetto L, Li L, Hernandez FP, Sandri-Goldin RM. Escudero-Paunetto L, et al. Virology. 2010 Jun 5;401(2):155-64. doi: 10.1016/j.virol.2010.02.023. Epub 2010 Mar 12. Virology. 2010. PMID: 20227104 Free PMC article. - Characterization and cloning of the human splicing factor 9G8: a novel 35 kDa factor of the serine/arginine protein family.
Cavaloc Y, Popielarz M, Fuchs JP, Gattoni R, Stévenin J. Cavaloc Y, et al. EMBO J. 1994 Jun 1;13(11):2639-49. doi: 10.1002/j.1460-2075.1994.tb06554.x. EMBO J. 1994. PMID: 8013463 Free PMC article. - SRp20: an overview of its role in human diseases.
Corbo C, Orrù S, Salvatore F. Corbo C, et al. Biochem Biophys Res Commun. 2013 Jun 21;436(1):1-5. doi: 10.1016/j.bbrc.2013.05.027. Epub 2013 May 16. Biochem Biophys Res Commun. 2013. PMID: 23685143 Review. - Nuclear export of messenger RNA.
Izaurralde E. Izaurralde E. Results Probl Cell Differ. 2002;35:133-50. doi: 10.1007/978-3-540-44603-3_7. Results Probl Cell Differ. 2002. PMID: 11791404 Review. No abstract available.
Cited by
- SRSF7 and SRSF3 depend on RNA sequencing motifs and secondary structures to regulate Microprocessor.
Le MN, Nguyen TD, Nguyen TA. Le MN, et al. Life Sci Alliance. 2023 Feb 7;6(4):e202201779. doi: 10.26508/lsa.202201779. Print 2023 Apr. Life Sci Alliance. 2023. PMID: 36750366 Free PMC article. - Dynamic nucleocytoplasmic shuttling of an Arabidopsis SR splicing factor: role of the RNA-binding domains.
Rausin G, Tillemans V, Stankovic N, Hanikenne M, Motte P. Rausin G, et al. Plant Physiol. 2010 May;153(1):273-84. doi: 10.1104/pp.110.154740. Epub 2010 Mar 17. Plant Physiol. 2010. PMID: 20237019 Free PMC article. - ATP is required for interactions between UAP56 and two conserved mRNA export proteins, Aly and CIP29, to assemble the TREX complex.
Dufu K, Livingstone MJ, Seebacher J, Gygi SP, Wilson SA, Reed R. Dufu K, et al. Genes Dev. 2010 Sep 15;24(18):2043-53. doi: 10.1101/gad.1898610. Genes Dev. 2010. PMID: 20844015 Free PMC article. - The cellular DExD/H-box RNA-helicases UAP56 and URH49 exhibit a CRM1-independent nucleocytoplasmic shuttling activity.
Thomas M, Lischka P, Müller R, Stamminger T. Thomas M, et al. PLoS One. 2011;6(7):e22671. doi: 10.1371/journal.pone.0022671. Epub 2011 Jul 22. PLoS One. 2011. PMID: 21799930 Free PMC article. - SR proteins are NXF1 adaptors that link alternative RNA processing to mRNA export.
Müller-McNicoll M, Botti V, de Jesus Domingues AM, Brandl H, Schwich OD, Steiner MC, Curk T, Poser I, Zarnack K, Neugebauer KM. Müller-McNicoll M, et al. Genes Dev. 2016 Mar 1;30(5):553-66. doi: 10.1101/gad.276477.115. Genes Dev. 2016. PMID: 26944680 Free PMC article.
References
- Bashford D, Case DA (2000) Generalized born models of macromolecular solvation effects. Annu Rev Phys Chem 51: 129–152 - PubMed
- Bax A, Grzesiek S (1993) Methodological advances in protein NMR. Acc Chem Res 26: 131–138
- Bourgeois CF, Lejeune F, Stevenin J (2004) Broad specificity of SR (serine/arginine) proteins in the regulation of alternative splicing of pre-messenger RNA. Prog Nucleic Acid Res Mol Biol 78: 37–88 - PubMed
- Case DA, Pearlman DA, Caldwell JW, Cheatham TE III, Wang J, Ross WS, Simmerling CL, Darden TA, Merz KM, Stanton RV, Cheng AL, Vincent JJ, Crowley M, Tsui V, Gohlke H, Radmer RJ, Duan Y, Pitera J, Massova I, Seibel GL, Singh UC, Weiner PK, Kollman PA (2002) AMBER 7. University of California, San Francisco
Publication types
MeSH terms
Substances
Grants and funding
- BB/D010519/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- MC_U117533887/MRC_/Medical Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous