Cap-free structure of eIF4E suggests a basis for conformational regulation by its ligands - PubMed (original) (raw)
Cap-free structure of eIF4E suggests a basis for conformational regulation by its ligands
Laurent Volpon et al. EMBO J. 2006.
Abstract
The activity of the eukaryotic translation initiation factor eIF4E is modulated through conformational response to its ligands. For example, eIF4G and eIF4E-binding proteins (4E-BPs) modulate cap affinity, and thus physiological activity of eIF4E, by binding a site distal to the 7-methylguanosine cap-binding site. Further, cap binding substantially modulates eIF4E's affinity for eIF4G and the 4E-BPs. To date, only cap-bound eIF4E structures were reported. In the absence of structural information on the apo form, the molecular underpinnings of this conformational response mechanism cannot be established. We report here the first cap-free eIF4E structure. Apo-eIF4E exhibits structural differences in the cap-binding site and dorsal surface relative to cap-eIF4E. Analysis of structure and dynamics of apo-eIF4E, and changes observed upon ligand binding, reveal a molecular basis for eIF4E's conformational response to these ligands. In particular, alterations in the S4-H4 loop, distal to either the cap or eIF4G binding sites, appear key to modulating these effects. Mutation in this loop mimics these effects. Overall, our studies have important implications for the regulation of eIF4E.
Figures
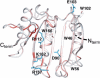
Figure 1
Crystal structure of the murine eIF4E/7-methyl-GDP–eIF4G peptide ternary complex (PDB 1EJH).
Figure 2
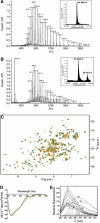
Mass spectrum of the apo- (A) and m7GDP-bound (B) forms of wild-type eIF4E. ES-MS spectra plotting ion abundance as a function of the mass/charge ratio. Insets: hypermass reconstruction of the spectrum. The low molecular weight peak at 458 is consistent with the presence of the m7GDP (457.23 Da). For more details, see figure legend in Supplementary Figure 3. Clearly, no cap-bound eIF4E is present in the apo sample. (C) Superposition of the 1H-15N HSQC spectrum of the apo-eIF4E (orange) and the m7GDP-bound eIF4E (green). (D) The far-UV CD spectra of apo-eIF4E (orange) and m7GDP–eIF4E (green). (E) Fluorescence emission of wild-type eIF4E in the presence of increasing concentrations of m7GDP (continuous) and intrinsic fluorescence of m7GDP in the absence of eIF4E (dashed). The different m7GDP concentrations (μM) are shown on the curves and fit is shown in Supplementary Figure 2.
Figure 3
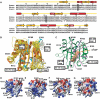
Structural comparison between apo- and cap-bound eIF4E. (A) Sequence alignments of eIF4E (Scerv., Saccharomyces cerevisiae). The secondary structural elements were assigned from the apo structure. W56 and W102 residues are boxed. (B) Superposition of the 10 lowest-energy NMR structures. Side chains of W56 and W102 are shown. (C) Crystal structure of human eIF4E bound to m7GDP (PDB 1EJ1). This structure contains density for residues 36–207 and 213–217. The side chains of residues that interact with the cap are shown. (D–G) Potential map of the surface of apo-eIF4E (D, F) and cap-eIF4E (E, G) calculated with MOLMOL. The orientation of (D) and (E) are the same as that of (B) and (C) (cap-binding site) while (F) and (G) represent a 180° rotation along a vertical axis compared with (D) and (E) (eIF4G binding site). A number of residues of interest are indicated on the structures (see text).
Figure 4
(A) hNOEs for backbone amide nitrogens of the apo-eIF4E (black) and m7GDP–eIF4E (red) measured at a proton frequency of 600 MHz. The cap (orange) and eIF4G (purple) binding sites on eIF4E are shown. (B) Motions associated with cap binding in the W56 and W102 loops. Four structures of the NMR ensemble of the apo-eIF4E (yellow) were superimposed with the cap structure (green), and loops containing W56 (S1–S2) and W102 (S3–S4) were highlighted for clarity. (C–F) Chemical shift perturbation of backbone 1HN and 15N resonances (color-coded) between the apo-eIF4E and m7GDP–eIF4E (C, D), and between the apo-eIF4E and apo-eIF4E-eIF4G peptide (E, F). These perturbations were mapped onto the apo-eIF4E structure. The orientation of (D) and (F) represent a 180° rotation along a vertical axis compared with panels (C) and (E), respectively. Analysis of ligand-induced shifts was performed by applying the Pythagorean theorem to weighted chemical shifts: Δδ (1H,15N)={Δδ(1H)2+0.2 × Δδ(15N)2}1/2, where Δδ(1H) and Δδ(15N) are the chemical-shift differences of the amide proton and nitrogen, respectively (Grzesiek et al, 1996; Pellecchia et al, 1999). These deviations are also shown for the indole Hɛ1 of the Trp residues.
Figure 5
Chemical shift differences between the apo and the ternary complex with either the cap-eIF4E (A), or the eIF4E-4G peptide (B) as binary complexes. The right panels represent the chemical shift differences for the eight Trp indoles HN. Positions indicated with arrows show proline residues (black), E103 which is not assigned in cap-eIF4E (purple) or peaks from which no data were collected due to overlap (green). Regions located on the cap (orange) and eIF4G (purple) binding sides on eIF4E are shown. (C) Differences in chemical shift index of α-protons between the apo and the cap-bound form of eIF4E. The secondary-structure elements of the apo structure are indicated below.
Figure 6
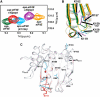
Prestructuring during cap and eIF4G binding. (A) Comparison of a section of the HSQC spectra of the apo-eIF4E (green), binary cap-eIF4E (orange) and ternary cap-eIF4E–eIF4G peptide (red). Assignments are indicated for the backbone HN (cyan) and the Trp indoles HN (blue). Chemical shifts for peaks were scored most highly (red, e.g. indole of W166 in part A) when their chemical shifts were intermediate between apo and ternary complexes upon addition of either the cap (B) or eIF4G (C).
Figure 7
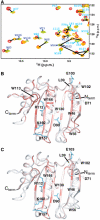
The eIF4E (K119A) mutant. (A) Superposition of 2D HSQC spectra showing residue R157 in the different complexes, together with the apo-eIF4E (K119A) mutant. (B) Structural differences between the apo- (orange) and the cap-eIF4E (green) around the residue K119 (see text for details). (C) Chemical shift perturbation of backbone 1HN and 15N resonances (color-coded) between apo-eIF4E and apo-eIF4E K119A. The deviations (in p.p.m.) were quantified with the same method as in Figure 4. K119 is shown in red.
Figure 8
Buffer effect on apo-eIF4E. Chemical shift perturbation of backbone 1HN and 15N resonances (color-coded) between apo-eIF4E in 50 mM phosphate pH 7.4, 100 mM NaCl and 20 mM HEPES pH 7.4, 100 mM NaCl. The deviations were quantified as in Figure 4. Side chains of some residues involved in cap binding are shown in blue.
Similar articles
- Structural characterization of the Z RING-eIF4E complex reveals a distinct mode of control for eIF4E.
Volpon L, Osborne MJ, Capul AA, de la Torre JC, Borden KL. Volpon L, et al. Proc Natl Acad Sci U S A. 2010 Mar 23;107(12):5441-6. doi: 10.1073/pnas.0909877107. Epub 2010 Mar 8. Proc Natl Acad Sci U S A. 2010. PMID: 20212144 Free PMC article. - The Structures of eIF4E-eIF4G Complexes Reveal an Extended Interface to Regulate Translation Initiation.
Grüner S, Peter D, Weber R, Wohlbold L, Chung MY, Weichenrieder O, Valkov E, Igreja C, Izaurralde E. Grüner S, et al. Mol Cell. 2016 Nov 3;64(3):467-479. doi: 10.1016/j.molcel.2016.09.020. Epub 2016 Oct 20. Mol Cell. 2016. PMID: 27773676 - Evaluating the conformation and binding interface of cap-binding proteins and complexes via ultraviolet photodissociation mass spectrometry.
O'Brien JP, Mayberry LK, Murphy PA, Browning KS, Brodbelt JS. O'Brien JP, et al. J Proteome Res. 2013 Dec 6;12(12):5867-77. doi: 10.1021/pr400869u. Epub 2013 Nov 14. J Proteome Res. 2013. PMID: 24200290 - The emerging roles of translation factor eIF4E in the nucleus.
Strudwick S, Borden KL. Strudwick S, et al. Differentiation. 2002 Mar;70(1):10-22. doi: 10.1046/j.1432-0436.2002.700102.x. Differentiation. 2002. PMID: 11963652 Review. - The Eukaryotic Translation Initiation Factor 4E (eIF4E) as a Therapeutic Target for Cancer.
Karaki S, Andrieu C, Ziouziou H, Rocchi P. Karaki S, et al. Adv Protein Chem Struct Biol. 2015;101:1-26. doi: 10.1016/bs.apcsb.2015.09.001. Epub 2015 Oct 23. Adv Protein Chem Struct Biol. 2015. PMID: 26572974 Free PMC article. Review.
Cited by
- Synthesis of N²-modified 7-methylguanosine 5'-monophosphates as nematode translation inhibitors.
Piecyk K, Davis RE, Jankowska-Anyszka M. Piecyk K, et al. Bioorg Med Chem. 2012 Aug 1;20(15):4781-9. doi: 10.1016/j.bmc.2012.05.078. Epub 2012 Jun 10. Bioorg Med Chem. 2012. PMID: 22748379 Free PMC article. - Structural studies of the eIF4E-VPg complex reveal a direct competition for capped RNA: Implications for translation.
Coutinho de Oliveira L, Volpon L, Rahardjo AK, Osborne MJ, Culjkovic-Kraljacic B, Trahan C, Oeffinger M, Kwok BH, Borden KLB. Coutinho de Oliveira L, et al. Proc Natl Acad Sci U S A. 2019 Nov 26;116(48):24056-24065. doi: 10.1073/pnas.1904752116. Epub 2019 Nov 11. Proc Natl Acad Sci U S A. 2019. PMID: 31712417 Free PMC article. - Structural insights into parasite eIF4E binding specificity for m7G and m2,2,7G mRNA caps.
Liu W, Zhao R, McFarland C, Kieft J, Niedzwiecka A, Jankowska-Anyszka M, Stepinski J, Darzynkiewicz E, Jones DN, Davis RE. Liu W, et al. J Biol Chem. 2009 Nov 6;284(45):31336-49. doi: 10.1074/jbc.M109.049858. Epub 2009 Aug 26. J Biol Chem. 2009. PMID: 19710013 Free PMC article. - DEAD Box Protein Family Member DDX28 Is a Negative Regulator of Hypoxia-Inducible Factor 2α- and Eukaryotic Initiation Factor 4E2-Directed Hypoxic Translation.
Evagelou SL, Bebenek O, Specker EJ, Uniacke J. Evagelou SL, et al. Mol Cell Biol. 2020 Feb 27;40(6):e00610-19. doi: 10.1128/MCB.00610-19. Print 2020 Feb 27. Mol Cell Biol. 2020. PMID: 31907278 Free PMC article. - Dynamic recognition of the mRNA cap by Saccharomyces cerevisiae eIF4E.
O'Leary SE, Petrov A, Chen J, Puglisi JD. O'Leary SE, et al. Structure. 2013 Dec 3;21(12):2197-207. doi: 10.1016/j.str.2013.09.016. Epub 2013 Oct 31. Structure. 2013. PMID: 24183571 Free PMC article.
References
- Blachut-Okrasinska E, Bojarska E, Niedzwiecka A, Chlebicka L, Darzynkiewicz E, Stolarski R, St pinski J, Antosiewicz JM (2000) Stopped-flow and Brownian dynamics studies of electrostatic effects in the kinetics of binding of 7-methyl-GpppG to the protein eIF4E. Eur Biophys J 29: 487–498 - PubMed
- Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, Read RJ, Rice LM, Simonson T, Warren GL (1998) Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr D 54 (Part 5): 905–921 - PubMed
- Cai A, Jankowska-Anyszka M, Centers A, Chlebicka L, Stepinski J, Stolarski R, Darzynkiewicz E, Rhoads RE (1999) Quantitative assessment of mRNA cap analogues as inhibitors of in vitro translation. Biochemistry 38: 8538–8547 - PubMed
- Carberry SE, Rhoads RE, Goss DJ (1989) A spectroscopic study of the binding of m7GTP and m7GpppG to human protein synthesis initiation factor 4E. Biochemistry 28: 8078–8083 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous