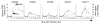Virus-induced type I IFN stimulates generation of immunoproteasomes at the site of infection - PubMed (original) (raw)
. 2006 Nov;116(11):3006-14.
doi: 10.1172/JCI29832. Epub 2006 Oct 12.
Affiliations
- PMID: 17039255
- PMCID: PMC1592549
- DOI: 10.1172/JCI29832
Virus-induced type I IFN stimulates generation of immunoproteasomes at the site of infection
Eui-Cheol Shin et al. J Clin Invest. 2006 Nov.
Abstract
IFN-gamma is known as the initial and primary inducer of immunoproteasomes during viral infections. We now report that type I IFN induced the transcription and translation of immunoproteasome subunits, their incorporation into the proteasome complex, and the generation of an immunoproteasome-dependent CD8 T cell epitope in vitro and provide in vivo evidence that this mechanism occurs prior to IFN-gamma responses at the site of viral infection. Type I IFN-mediated generation of immunoproteasomes was initiated by either poly(I:C) or HCV RNA in human hepatoma cells and was inhibited by neutralization of type I IFN. In serial liver biopsies of chimpanzees with acute HCV infection, increases in immunoproteasome subunit mRNA preceded intrahepatic IFN-gamma responses by several weeks, instead coinciding with intrahepatic type I IFN responses. Thus, viral RNA-induced innate immune responses regulate the antigen-processing machinery, which occurs prior to the detection of IFN-gamma at the site of infection. This mechanism may contribute to the high effectiveness (95%) of type I IFN-based therapies if administered early during HCV infection.
Figures
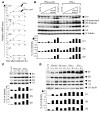
Figure 1. Type I IFN induces the expression of immunoproteasome subunits in vitro.
(A) Huh-7 cells were treated with 3 ng/ml IFN-α–con1 (squares) or 10 ng/ml (200 U/ml) IFN-γ (triangles) for the indicated time periods, after which the mRNA levels of immunoproteasome subunits were quantified by real-time PCR. mRNA levels were normalized to endogenous references (GAPDH and β-actin) and expressed as fold increase over pretreatment levels. The β7 subunit was measured as a control. (B) Huh-7 cells were treated with the indicated doses of IFN-α–con1 or IFN-γ for 48 hours, and Western blot analysis was performed to detect immunoproteasome subunits. The α4 subunit was analyzed as a control. (C) Primary human hepatocytes were treated with 3 ng/ml IFN-α–con1 or 10 ng/ml IFN-γ for the indicated time periods prior to Western blot analysis. (D) Huh-7 cells were treated with 1.8 ng/ml (500 U/ml) IFN-α2a, 3 ng/ml IFN-α–con1, 3 ng/ml IFN-β, or 10 ng/ml IFN-γ for the indicated time periods, and Western blot analysis was performed to detect immunoproteasome subunits. The density of the Western blot bands was quantified and expressed as percent of the density of the α4 control band.
Figure 2. Type I IFN–induced immunoproteasomes exhibit the typical structure and function of IFN-γ–induced immunoproteasomes.
(A) Huh-7 cells were treated with 3 ng/ml IFN-α–con1 or 10 ng/ml IFN-γ for 36 hours, and the 20S proteasome complex was biochemically isolated from cell lysates. Isolated 20S proteasomes were analyzed by 2-dimensional gel electropheresis and Coomassie blue staining. The locations of β1i and β5i are indicated in the insets to the right (magnification, ×2). (B) Huh-7 cells were treated with 3 ng/ml IFN-α–con1 or 10 ng/ml IFN-γ for 36 hours and labeled with 35S-methionine. The 20S proteasome complex was immunoprecipitated and analyzed by 2-dimensional gel electropheresis and autoradiography. The locations of β1, β5, β1i, and β5i are indicated. (C) Biochemically isolated 20S proteasome complexes as shown in A were subsequently incubated with the precursor substrate HBcore131–162 for the indicated time periods. In vitro digests were analyzed by HPLC and mass spectrometry at the indicated time points for the presence of the β5i-dependent HBcore141–151 and the β5i-independent HBcore131–140. Type I IFN–induced immunoproteasomes displayed β5i-dependent proteolytic activity.
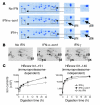
Figure 3. Intracellular dsRNA induces the expression of immunoproteasome subunits by the secretion of type I IFN.
Huh-7 cells were transfected with 10 μg poly(I:C) (squares) or 10 μg pcDNA3.1 plasmid DNA (inverted triangles), treated with 10 μg/ml extracellular poly(I:C) (triangles), or were mock transfected (circles). (A) Quantitation of IFN-β and 2,5-OAS-1 mRNA by real-time PCR. Bars (indicating secreted IFN-β) are shown only for the poly(I:C)-transfected Huh-7 cells because no secreted IFN-β was detectable in the control cultures of DNA-transfected Huh-7 cells or Huh-7 cells treated extracellularly with poly(I:C). (B) Quantitation of mRNA levels of immunoproteasome subunits β1i, β5i, β2i and constitutive subunit β7 by real-time PCR. (C–E) Detection of immunoproteasome subunits β1i, β5i, and β2i by Western blot analysis. (C and D) Huh-7 cells were treated with IFN-α and IFN-γ as well as (C) treated with extracellular DNA and poly(I:C) or (D) transfected with DNA and poly(I:C). (E) Four hours after poly(I:C) transfection of Huh-7 cells, 5,000 U/ml neutralizing anti–IFN-α and 2,000 U/ml neutralizing anti–IFN-β, alone or in combination, as well as 1 μg/ml VV B18R protein were added into culture and incubated for 48 hours, and Western blot was performed to detect immunoproteasome subunits. Anti–IFN-α/β, combination of IFN-α– and IFN-β–neutralizing antibodies. The density of the Western blot bands was quantified and expressed as percent of the density of the α4 control band.
Figure 4. HCV RNA induces the expression of immunoproteasome subunits.
Huh-7 cells were transfected with 2 μg in vitro transcribed H77 HCV RNA (squares) or were mock transfected (circles). (A) mRNA levels of IFN-β and 2,5-OAS-1 were determined by real-time PCR. Secreted IFN-β (bars in left panel) was detectable by ELISA only in the supernatants of HCV RNA-transfected Huh-7 cells, not in supernatants of mock-transfected Huh-7 cells (not shown). (B) mRNA levels of immunoproteasome subunits β1i, β5i, and β2i and the constitutive subunit β7 as determined by real-time PCR.
Figure 6. Induction of immunoproteasome subunits in the liver precedes infiltration of CD8 T cells.
CD8β mRNA levels were quantified in serial liver biopsies of chimpanzees with acute HCV infection. CD8β mRNA amounts (squares) correlated closely with IFN-γ mRNA amounts (gray lines; values reproduced from Figure 5D for reference).
Figure 5. Immunoproteasome subunit mRNA levels increase early in acute HCV infection and in temporal relation to type I IFN responses.
Five chimpanzees (Ch6455, Ch6461, Ch1606, Ch6475, and Ch6411) were studied prospectively during acute HCV infection. (A) Serum ALT levels (open squares) and serum HCV RNA titers (black diamonds) have previously been reported (44) and are presented for reference purposes. (B–G) Serial liver biopsies were analyzed for mRNA levels of (B) β1i (squares) and β7 (triangles); (C) β5i (squares) and β2i (triangles); (D) IFN-γ (squares) and CXCL9 (triangles); (E) TNF-α; (F) 2,5-OAS-1; and (G) IFN-α2 (black line), IFN-α14 (dashed line), IFN-α21 dotted line), and IFN-β (gray line). mRNA levels were normalized to endogenous references (GAPDH and β-actin) and expressed as fold increase over preinfection levels. In Ch1606, the relative mRNA level of IFN-α21 was 36.9 at week 4. Vertical dashed lines separate the time intervals prior to the first major (greater than 2-fold) increase of IFN-γ or TNF-α mRNA levels. NT, not tested due to the shortage of cDNA.
Similar articles
- Proteasome activator and antigen-processing aminopeptidases are regulated by virus-induced type I interferon in the hepatitis C virus-infected liver.
Shin EC, Seifert U, Urban S, Truong KT, Feinstone SM, Rice CM, Kloetzel PM, Rehermann B. Shin EC, et al. J Interferon Cytokine Res. 2007 Dec;27(12):985-90. doi: 10.1089/jir.2007.0039. J Interferon Cytokine Res. 2007. PMID: 18184038 - Immunoproteasome induction is suppressed in hepatitis C virus-infected cells in a protein kinase R-dependent manner.
Oh IS, Textoris-Taube K, Sung PS, Kang W, Gorny X, Kähne T, Hong SH, Choi YJ, Cammann C, Naumann M, Kim JH, Park SH, Yoo OJ, Kloetzel PM, Seifert U, Shin EC. Oh IS, et al. Exp Mol Med. 2016 Nov 11;48(11):e270. doi: 10.1038/emm.2016.98. Exp Mol Med. 2016. PMID: 27833096 Free PMC article. - Delayed induction, not impaired recruitment, of specific CD8⁺ T cells causes the late onset of acute hepatitis C.
Shin EC, Park SH, Demino M, Nascimbeni M, Mihalik K, Major M, Veerapu NS, Heller T, Feinstone SM, Rice CM, Rehermann B. Shin EC, et al. Gastroenterology. 2011 Aug;141(2):686-95, 695.e1. doi: 10.1053/j.gastro.2011.05.006. Epub 2011 May 18. Gastroenterology. 2011. PMID: 21699897 Free PMC article. - Innate and adaptive immune responses in HCV infections.
Heim MH, Thimme R. Heim MH, et al. J Hepatol. 2014 Nov;61(1 Suppl):S14-25. doi: 10.1016/j.jhep.2014.06.035. Epub 2014 Nov 3. J Hepatol. 2014. PMID: 25443342 Review. - Activation of endogenous type I IFN signaling contributes to persistent HCV infection.
Li Y, Li S, Duan X, Liu B, Yang C, Zeng P, McGilvray I, Chen L. Li Y, et al. Rev Med Virol. 2014 Sep;24(5):332-42. doi: 10.1002/rmv.1795. Epub 2014 May 8. Rev Med Virol. 2014. PMID: 24806972 Review.
Cited by
- Immunological aspects of antiviral therapy of chronic hepatitis B virus and hepatitis C virus infections.
Rehermann B, Bertoletti A. Rehermann B, et al. Hepatology. 2015 Feb;61(2):712-21. doi: 10.1002/hep.27323. Hepatology. 2015. PMID: 25048716 Free PMC article. Review. - Successful Interferon-Free Therapy of Chronic Hepatitis C Virus Infection Normalizes Natural Killer Cell Function.
Serti E, Chepa-Lotrea X, Kim YJ, Keane M, Fryzek N, Liang TJ, Ghany M, Rehermann B. Serti E, et al. Gastroenterology. 2015 Jul;149(1):190-200.e2. doi: 10.1053/j.gastro.2015.03.004. Epub 2015 Mar 6. Gastroenterology. 2015. PMID: 25754160 Free PMC article. - Immune responses and immunopathology in acute and chronic viral hepatitis.
Shin EC, Sung PS, Park SH. Shin EC, et al. Nat Rev Immunol. 2016 Aug;16(8):509-23. doi: 10.1038/nri.2016.69. Epub 2016 Jul 4. Nat Rev Immunol. 2016. PMID: 27374637 Review. - Reduction in ATP levels triggers immunoproteasome activation by the 11S (PA28) regulator during early antiviral response mediated by IFNβ in mouse pancreatic β-cells.
Freudenburg W, Gautam M, Chakraborty P, James J, Richards J, Salvatori AS, Baldwin A, Schriewer J, Buller RM, Corbett JA, Skowyra D. Freudenburg W, et al. PLoS One. 2013;8(2):e52408. doi: 10.1371/journal.pone.0052408. Epub 2013 Feb 1. PLoS One. 2013. PMID: 23383295 Free PMC article. - Liver natural killer and natural killer T cells: immunobiology and emerging roles in liver diseases.
Gao B, Radaeva S, Park O. Gao B, et al. J Leukoc Biol. 2009 Sep;86(3):513-28. doi: 10.1189/JLB.0309135. J Leukoc Biol. 2009. PMID: 19542050 Free PMC article. Review.
References
- Rock K.L., et al. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell. 1994;78:761–771. - PubMed
- Van den Eynde B.J., Morel S. Differential processing of class-I-restricted epitopes by the standard proteasome and the immunoproteasome. Curr. Opin. Immunol. 2001;13:147–153. - PubMed
- Kloetzel P.M. Antigen processing by the proteasome. Nat. Rev. Mol. Cell Biol. 2001;2:179–187. - PubMed
- Fruh K., Yang Y. Antigen presentation by MHC class I and its regulation by interferon gamma. Curr. Opin. Immunol. 1999;11:76–81. - PubMed
- Foss G.S., Larsen F., Solheim J., Prydz H. Constitutive and interferon-gamma-induced expression of the human proteasome subunit multicatalytic endopeptidase complex-like 1. Biochim. Biophys. Acta. 1998;1402:17–28. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials