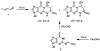Analysis of crotonaldehyde- and acetaldehyde-derived 1,n(2)-propanodeoxyguanosine adducts in DNA from human tissues using liquid chromatography electrospray ionization tandem mass spectrometry - PubMed (original) (raw)
Analysis of crotonaldehyde- and acetaldehyde-derived 1,n(2)-propanodeoxyguanosine adducts in DNA from human tissues using liquid chromatography electrospray ionization tandem mass spectrometry
Siyi Zhang et al. Chem Res Toxicol. 2006 Oct.
Abstract
Crotonaldehyde, a mutagen and carcinogen, reacts with deoxyguanosine (dGuo) in DNA to generate a pair of diastereomeric 1,N(2)()-propanodeoxyguanosine adducts (Cro-dGuo, 2), which occur in (6S,8S) and (6R,8R) configurations. They can also be formed through the consecutive reaction of two acetaldehyde molecules with dGuo. Cro-dGuo adducts inhibit DNA synthesis and induce miscoding in human cells. Considering their potential role in carcinogenesis, we have developed a sensitive and specific liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS) method to explore the presence of Cro-dGuo adducts in DNA from various human tissues, such as liver, lung, and blood. DNA was isolated from human tissues and enzymatically hydrolyzed to deoxyribonucleosides. [(15)N(5)]Cro-dGuo was synthesized and used as an internal standard. The Cro-dGuo adducts were enriched from the hydrolysate by solid-phase extraction and analyzed by LC-ESI-MS/MS using selected reaction monitoring (SRM). This method allows the quantitation of the Cro-dGuo adducts at a concentration of 4 fmol/micromol dGuo, corresponding to about 1 adduct per 10(9) normal nucleosides starting with 1 mg of DNA, with high accuracy and precision. DNA from human liver, lung, and blood was analyzed. The Cro-dGuo adducts were detected more frequently in human lung DNA than in liver DNA but were not detected in DNA from blood. The results of this study provide quantified data on Cro-dGuo adducts in human tissues. The higher frequency of Cro-dGuo in lung DNA than in the other tissues investigated is potentially important and deserves further study.
Figures
Figure 1
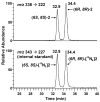
Chromatograms obtained upon LC-ESI-MS/MS analysis of 0.5 fmol standard Cro-dGuo (2) (top) and 10 fmol [15N5]Cro-dGuo ([15N5]2) (bottom). Peak areas were 4.9*104 for (6S, 8S)-2, 5.5*104 for (6R, 8R)-2, 1.1*106 for (6S, 8S)-[15N5]2, and 1.2*106 for (6R, 8R)-[15N5]2.
Figure 2
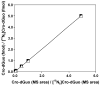
Calibration curves for Cro-dGuo (2, 0.5–50 fmol) and [15N5]Cro-dGuo ([15N5]2, 10 fmol): ■, (6S, 8S)-2, R2 = 1.0000;, ρ, (6R, 8R)-2, R2 = 1.0000.
Figure 3
Chromatograms obtained upon LC-ESI-MS/MS analysis of calf thymus DNA. Calf thymus DNA was enzymatically hydrolyzed, purified by SPE, and analyzed (panel A); or the eluants from SPE were treated with NaOH and NaBH4 and analyzed (panel B). Transitions of m/z 340 → m/z 224 and m/z 345 → m/z 229 correspond to the ring-opened products of the analyte and internal standard, N2_-(4-hydroxybut-2-yl)dGuo and [15N5]N2_-(4-hydroxybut-2-yl)dGuo, respectively. The early eluting peak was produced from (6_R, 8R)-2 and the late eluting peak from (6_S, 8S)-2.
Figure 4
Relationship of added to detected Cro-dGuo (2). Various amounts of standard adduct 2 were added to calf thymus DNA (0.91 mg) containing [15N5]2 and analyzed by the method described in the text. Adduct 2 in calf thymus DNA [9.80 fmol/mg DNA for (6S, 8S)-2 and 8.49 fmol/mg DNA for (6R, 8R)-2] was subtracted from each amount detected. Each point represents a triplicate measurement. A, (6S, 8S)-2, R2 = 0.9986; B, (6R, 8R)-2, R2 = 1.0000.
Figure 5
Chromatograms obtained upon LC-ESI-MS/MS-SRM analysis of DNA from human liver and lung. A and C, DNA from human liver; B, DNA from the same human liver as in A, to which 2 fmol of each diastereomer of Cro-dGuo was added; D, DNA from human lung.
Scheme 1
Formation of _1,N2_-propanodeoxyguanosine adducts in the reactions of crotonaldehyde or acetaldehyde with dGuo.
Similar articles
- DNA cross-link induced by trans-4-hydroxynonenal.
Huang H, Kozekov ID, Kozekova A, Wang H, Lloyd RS, Rizzo CJ, Stone MP. Huang H, et al. Environ Mol Mutagen. 2010 Jul;51(6):625-34. doi: 10.1002/em.20599. Environ Mol Mutagen. 2010. PMID: 20577992 Free PMC article. Review. - Detection and quantitation of acrolein-derived 1,N2-propanodeoxyguanosine adducts in human lung by liquid chromatography-electrospray ionization-tandem mass spectrometry.
Zhang S, Villalta PW, Wang M, Hecht SS. Zhang S, et al. Chem Res Toxicol. 2007 Apr;20(4):565-71. doi: 10.1021/tx700023z. Epub 2007 Mar 27. Chem Res Toxicol. 2007. PMID: 17385896 Free PMC article. - Identification of an acetaldehyde adduct in human liver DNA and quantitation as N2-ethyldeoxyguanosine.
Wang M, Yu N, Chen L, Villalta PW, Hochalter JB, Hecht SS. Wang M, et al. Chem Res Toxicol. 2006 Feb;19(2):319-24. doi: 10.1021/tx0502948. Chem Res Toxicol. 2006. PMID: 16485909 Free PMC article. - Quantitation by Liquid Chromatography-Nanoelectrospray Ionization-High-Resolution Tandem Mass Spectrometry of Multiple DNA Adducts Related to Cigarette Smoking in Oral Cells in the Shanghai Cohort Study.
Cheng G, Guo J, Wang R, Yuan JM, Balbo S, Hecht SS. Cheng G, et al. Chem Res Toxicol. 2023 Feb 20;36(2):305-312. doi: 10.1021/acs.chemrestox.2c00393. Epub 2023 Jan 31. Chem Res Toxicol. 2023. PMID: 36719849 Free PMC article. - Liquid chromatography-electrospray ionization-mass spectrometry: the future of DNA adduct detection.
Singh R, Farmer PB. Singh R, et al. Carcinogenesis. 2006 Feb;27(2):178-96. doi: 10.1093/carcin/bgi260. Epub 2005 Nov 4. Carcinogenesis. 2006. PMID: 16272169 Review.
Cited by
- Interplay between Cellular Metabolism and the DNA Damage Response in Cancer.
Moretton A, Loizou JI. Moretton A, et al. Cancers (Basel). 2020 Jul 25;12(8):2051. doi: 10.3390/cancers12082051. Cancers (Basel). 2020. PMID: 32722390 Free PMC article. Review. - Smoking and lung cancer--a new role for an old toxicant?
Hecht SS. Hecht SS. Proc Natl Acad Sci U S A. 2006 Oct 24;103(43):15725-6. doi: 10.1073/pnas.0607811103. Epub 2006 Oct 16. Proc Natl Acad Sci U S A. 2006. PMID: 17043212 Free PMC article. No abstract available. - Comprehensive Assessment of Oxidatively Induced Modifications of DNA in a Rat Model of Human Wilson's Disease.
Yu Y, Guerrero CR, Liu S, Amato NJ, Sharma Y, Gupta S, Wang Y. Yu Y, et al. Mol Cell Proteomics. 2016 Mar;15(3):810-7. doi: 10.1074/mcp.M115.052696. Epub 2015 Sep 11. Mol Cell Proteomics. 2016. PMID: 26362317 Free PMC article. - Insertion of dNTPs opposite the 1,N2-propanodeoxyguanosine adduct by Sulfolobus solfataricus P2 DNA polymerase IV.
Wang Y, Musser SK, Saleh S, Marnett LJ, Egli M, Stone MP. Wang Y, et al. Biochemistry. 2008 Jul 15;47(28):7322-34. doi: 10.1021/bi800152j. Epub 2008 Jun 19. Biochemistry. 2008. PMID: 18563918 Free PMC article. - DNA cross-link induced by trans-4-hydroxynonenal.
Huang H, Kozekov ID, Kozekova A, Wang H, Lloyd RS, Rizzo CJ, Stone MP. Huang H, et al. Environ Mol Mutagen. 2010 Jul;51(6):625-34. doi: 10.1002/em.20599. Environ Mol Mutagen. 2010. PMID: 20577992 Free PMC article. Review.
References
- Chung FL, Chen HJ, Nath RG. Lipid peroxidation as a potential endogenous source for the formation of exocyclic DNA adducts. Carcinogenesis. 1996;17:2105–2111. - PubMed
- Wang MY, Chung FL, Hecht SS. Identification of crotonaldehyde as a hepatic microsomal metabolite formed by α-hydroxylation of the carcinogen N-nitrosopyrrolidine. Chem Res Toxicol. 1988;1:28–31. - PubMed
- Neudecker T, Eder E, Deininger C, Henschler D. Crotonaldehyde is mutagenic in Salmonella typhimurium TA100. Environ Mol Mutagen. 1989;14:146–148. - PubMed
- Chung FL, Tanaka T, Hecht SS. Induction of liver tumors in F344 rats by crotonaldehyde. Cancer Res. 1986;46:1285–1289. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources