Role of seminal plasma in the anti-HIV-1 activity of candidate microbicides - PubMed (original) (raw)
Role of seminal plasma in the anti-HIV-1 activity of candidate microbicides
A Robert Neurath et al. BMC Infect Dis. 2006.
Abstract
Background: Evaluation of microbicides for prevention of HIV-1 infection in macaque models for vaginal infection has indicated that the concentrations of active compounds needed for protection by far exceed levels sufficient for complete inhibition of infection in vitro. These experiments were done in the absence of seminal plasma (SP), a vehicle for sexual transmission of the virus. To gain insight into the possible effect of SP on the performance of selected microbicides, their anti-HIV-1 activity in the presence, and absence of SP, was determined.
Methods: The inhibitory activity of compounds against the X4 virus, HIV-1 IIIB, and the R5 virus, HIV-1 BaL was determined using TZM-bl indicator cells and quantitated by measuring beta-galactosidase induced by infection. The virucidal properties of cellulose acetate 1,2-benzene-dicarboxylate (CAP), the only microbicide provided in water insoluble, micronized form, in the presence of SP was measured.
Results: The HIV-1 inhibitory activity of the polymeric microbicides, poly(naphthalene sulfonate), cellulose sulfate, carrageenan, CAP (in soluble form) and polystyrene sulfonate, respectively, was considerably (range approximately 4 to approximately 73-fold) diminished in the presence of SP (33.3%). Formulations of micronized CAP, providing an acidic buffering system even in the presence of an SP volume excess, effectively inactivated HIV-1 infectivity.
Conclusion: The data presented here suggest that the in vivo efficacy of polymeric microbicides, acting as HIV-1 entry inhibitors, might become at least partly compromised by the inevitable presence of SP. These possible disadvantages could be overcome by combining the respective polymers with acidic pH buffering systems (built-in for formulations of micronized CAP) or with other anti-HIV-1 compounds, the activity of which is not affected by SP, e.g. reverse transcriptase and zinc finger inhibitors.
Figures
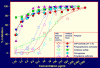
Figure 1
Inhibition of HIV-1 IIIB infection by negatively charged polymers in the presence (33.3%; v/v), and absence of seminal plasma, respectively. TZM-bl indicator cells and β-galactosidase readout were used for quantitative analysis of HIV-1 infection.
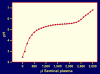
Figure 2
pH changes caused by addition of seminal plasma to 180 mg Aquateric. Increasing volumes of seminal plasma were added to 180 mg Aquateric, and pH was measured. Aquateric is a micronized form of CAP and consists of ≈ 67% CAP and ≈ 33% poloxamer + distilled acetylated monoglycerides. CAP formulations have been designed to contain 180 mg Aquateric per gram of formulation.
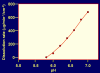
Figure 3
Dissolution rate of CAP as a function of pH. Data are derived from J. Spitael and R. Klinget: Solubility and dissolution rate of enteric polymers, Acta Pharmaceutica technologica 1979; S7:163–168 [56].
Figure 4
CAP solubility in seminal plasma-Aquateric formulation mixtures. Increasing volumes of seminal plasma were added to one ml of formulation 1 (see Methods section). The pH was measured; the mixtures were centrifuged to pellet most of Aquateric and CAP in the supernatant fluids was quantitated [25].
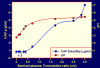
Figure 5
HIV-1 BaL inactivation in seminal plasma-Aquateric formulation mixtures. Increasing volumes of seminal plasma were added to aliquots of formulation 2 (see Methods section). After 5 min at 37°C, the samples were cooled in ice, and residual infectivity was measured. The decrease in extent of virus inactivation reflects both dilutions of the Aquateric formulation by seminal plasma and a concomitant pH increase. Samples diluted ≤ 8-fold were cytotoxic due to low pH, and the percentage of virus inactivation could not be determined.
Similar articles
- Anti-HIV-1 activity of anionic polymers: a comparative study of candidate microbicides.
Neurath AR, Strick N, Li YY. Neurath AR, et al. BMC Infect Dis. 2002 Nov 21;2:27. doi: 10.1186/1471-2334-2-27. Epub 2002 Nov 21. BMC Infect Dis. 2002. PMID: 12445331 Free PMC article. - Effect of topical microbicides on infectious human immunodeficiency virus type 1 binding to epithelial cells.
Roth S, Monsour M, Dowland A, Guenthner PC, Hancock K, Ou CY, Dezzutti CS. Roth S, et al. Antimicrob Agents Chemother. 2007 Jun;51(6):1972-8. doi: 10.1128/AAC.01358-06. Epub 2007 Apr 2. Antimicrob Agents Chemother. 2007. PMID: 17404008 Free PMC article. - Combination of candidate microbicides cellulose acetate 1,2-benzenedicarboxylate and UC781 has synergistic and complementary effects against human immunodeficiency virus type 1 infection.
Liu S, Lu H, Neurath AR, Jiang S. Liu S, et al. Antimicrob Agents Chemother. 2005 May;49(5):1830-6. doi: 10.1128/AAC.49.5.1830-1836.2005. Antimicrob Agents Chemother. 2005. PMID: 15855503 Free PMC article. - Clinical development of microbicides for the prevention of HIV infection.
D'Cruz OJ, Uckun FM. D'Cruz OJ, et al. Curr Pharm Des. 2004;10(3):315-36. doi: 10.2174/1381612043386374. Curr Pharm Des. 2004. PMID: 14754390 Review. - Microbicides: a new hope for HIV prevention.
Nutan; Gupta SK. Nutan, et al. Indian J Med Res. 2011 Dec;134(6):939-49. doi: 10.4103/0971-5916.92639. Indian J Med Res. 2011. PMID: 22310826 Free PMC article. Review.
Cited by
- Abolishing HIV-1 infectivity using a polypurine tract-specific G-quadruplex-forming oligonucleotide.
Voges M, Schneider C, Sinn M, Hartig JS, Reimer R, Hauber J, Moelling K. Voges M, et al. BMC Infect Dis. 2016 Jul 22;16:358. doi: 10.1186/s12879-016-1713-x. BMC Infect Dis. 2016. PMID: 27450669 Free PMC article. - Enhancement of HIV infection by cellulose sulfate.
Tao W, Richards C, Hamer D. Tao W, et al. AIDS Res Hum Retroviruses. 2008 Jul;24(7):925-9. doi: 10.1089/aid.2008.0043. AIDS Res Hum Retroviruses. 2008. PMID: 18627218 Free PMC article. - Human immunodeficiency virus type 1 nucleocapsid inhibitors impede trans infection in cellular and explant models and protect nonhuman primates from infection.
Wallace GS, Cheng-Mayer C, Schito ML, Fletcher P, Miller Jenkins LM, Hayashi R, Neurath AR, Appella E, Shattock RJ. Wallace GS, et al. J Virol. 2009 Sep;83(18):9175-82. doi: 10.1128/JVI.00820-09. Epub 2009 Jul 8. J Virol. 2009. PMID: 19587055 Free PMC article. - Female genital tract secretions and semen impact the development of microbicides for the prevention of HIV and other sexually transmitted infections.
Herold BC, Mesquita PM, Madan RP, Keller MJ. Herold BC, et al. Am J Reprod Immunol. 2011 Mar;65(3):325-33. doi: 10.1111/j.1600-0897.2010.00932.x. Epub 2010 Dec 12. Am J Reprod Immunol. 2011. PMID: 21143689 Free PMC article. Review. - Enzymatic triggered release of an HIV-1 entry inhibitor from prostate specific antigen degradable microparticles.
Clark MR, Aliyar HA, Lee CW, Jay JI, Gupta KM, Watson KM, Stewart RJ, Buckheit RW, Kiser PF. Clark MR, et al. Int J Pharm. 2011 Jul 15;413(1-2):10-18. doi: 10.1016/j.ijpharm.2011.04.004. Epub 2011 Apr 12. Int J Pharm. 2011. PMID: 21511017 Free PMC article.
References
- UNAIDS 2006 Report on the global AIDS epidemic. 2006.
- Boadi T, Schneider E, Chung S, Tsai L, Gettie A, Ratterree M, Blanchard J, Neurath AR, Cheng-Mayer C. Cellulose acetate 1,2-benzenedicarboxylate protects against challenge with pathogenic X4 and R5 simian-human immunodeficiency viruses. AIDS. 2005;19:1587–1594. doi: 10.1097/01.aids.0000186020.24426.62. - DOI - PubMed
- Otten RA, Adams DR, Kim CN, Jackson E, Pullium JK, Lee K, Grohskopf LA, Monsour M, Butera S, Folks TM. Multiple vaginal exposures to low doses of R5 simian-human immunodeficiency virus: Strategy to study HIV preclinical interventions in nonhuman primates. J Infect Dis. 2005;191:164–173. doi: 10.1086/426452. - DOI - PubMed
- Weber J, Nunn A, O'Connor T, Jeffries D, Kitchen V, McCormack S, Stott J, Almond N, Stone A, Darbyshire J. 'Chemical condoms' for the prevention of HIV infection: evaluation of novel agents against SHIV89.6PD in vitro and in vivo. AIDS. 2001;15:1563–1568. doi: 10.1097/00002030-200108170-00014. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous